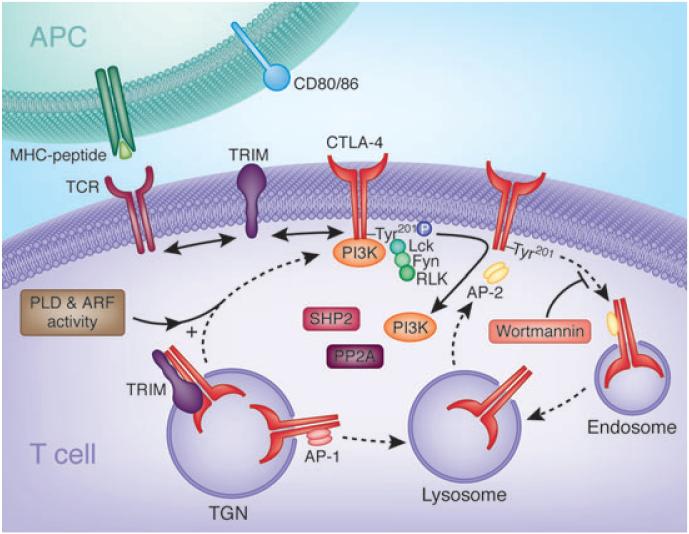
Fig. 2. Regulation of CTLA-4 surface expression and internalization.
Newly synthesized CTLA-4 binds to the transmembrane adapter TRIM in the TGN promoting the formation of CTLA-4-containing vesicles and their transport to the cell surface. On the cell surface, CTLA-4 and TRIM no longer associate allowing TRIM to interact with other receptors, possibly the TCR complex. CTLA-4 externalization is also dependent on general factors such as PLD and GTPase ARF-1. Shuttling to the lysosomal compartment from the TGN occurs due to adapter AP-1 binding to GVY201VKM motif in CTLA-4. On the surface, CTLA-4 becomes phosphorylated on the Y201VKM by kinases Lck, Fyn, and Rlk leading to the association of PI3K and possibly other proteins. Phosphorylation retards internationalization. Dephosphorylation allows binding to the clathrin adapter AP-2 to the GVY201VKM motif and rapid internalization to endosomes and lysosomes. Upon T-cell activation, CTLA-4 enriched lysosomes (and endosomes) are recycled to the cell surface. This process involves Wortmannin-sensitive lipid kinases but not PI3K (174). CTLA-4, cytotoxic T-lymphocyte antigen-4; TRIM, T-cell receptor-interacting molecule; TGN, trans-Golgi network; TCR, T-cell receptor; PLD, phospholipase D; ARF, adenosine diphosphate ribosylation factor; AP, adapter protein; PI3K, phosphatidylinositol 3-kinase.