Fig. 1.
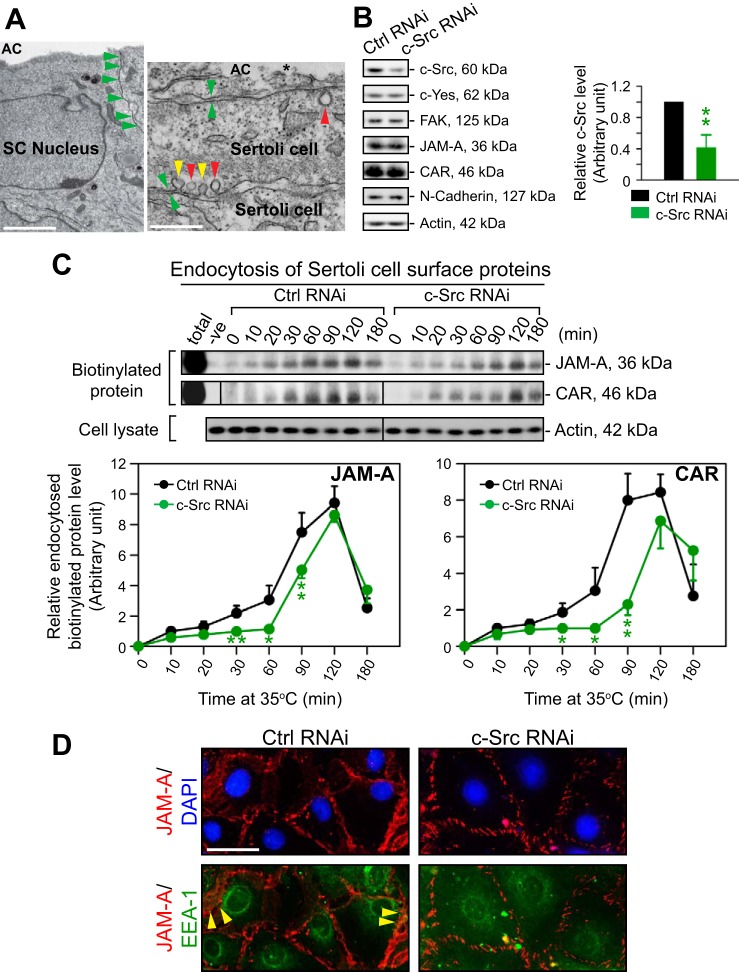
Effects of c-Src knockdown by RNAi on the kinetics of endocytosis of integral membrane proteins at the Sertoli cell blood-testis barrier (BTB) in vitro. A: Sertoli cells (SC) cultured in vitro for 4 days were processed for electron microscopy (EM). Left: specialized junctions that constitute the BTB were found at the cell-cell interface when cells were cultured on Matrigel-coated bicameral units (annotated by green arrowheads); the apical compartment (AC) was noted, and typical SC nucleus was also detected. Right: in these SC cultures, endocytic vesicle-mediated trafficking was also detected and annotated by yellow and red arrowheads, illustrating that the endocytic vesicle (also known as basal tubulobulbar complex in the testis) remained attached or had just been detached and released from the SC plasma membrane, respectively, confirming the presence of endocytic vesicle-mediated trafficking. *Microvillus that is a typical ultrastructure when SC are cultured in vitro. Scale bar, 1 μm on left and 0.2 μm on right. B: SC were cultured for 2 days with an established tight junction (TJ)-permeability barrier before they were transfected with RNAi duplexes for 24 h. About 36 h thereafter, cells were harvested for immunoblot analysis. It was noted that c-Src was knocked down by ≥60% (left) without apparent off-target effects in potentially related signaling pathways since the levels of c-Yes, FAK, and other BTB proteins JAM-A, CAR, and N-cadherin were not perturbed following c-Src silencing. These findings were representative data of 3 independent experiments. β-Actin served as the protein loading control. Each bar in the histogram (right) is the mean ± SD of n = 3 experiments. C: when the kinetics of protein endocytosis in cells transfected with nontargeting control duplexes vs. specific c-Src duplexes was compared, the knockdown of c-Src by RNAi was found to decelerate the kinetics of endocytosis of JAM-A and CAR significantly. Each data point is the mean ± SD of n = 4 experiments. D: effects of c-Src silencing that impeded the endocytosis of BTB integral membrane proteins JAM-A and CAR were corroborated in this dual-labeled immunofluorescence analysis in which cells transfected with nontargeting control duplexes, endocytosed JAM-A protein partially colocalized with EEA-1 [early endosome antigen-1; a endocytic marker (see yellow arrowheads)], and fewer endocytosed proteins, such as JAM-A, were found to colocalize with EEA-1 following c-Src knockdown, since more EEA-1 was found in SC cytosol instead of near the cell surface as in control cells. These micrographs are representative findings of 3 experiments. Bar, 20 μm (which applies to other micrographs). In B and C, *P < 0.05 and **P < 0.01. DAPI, 4′,6-diamidino-2-phenylindole.