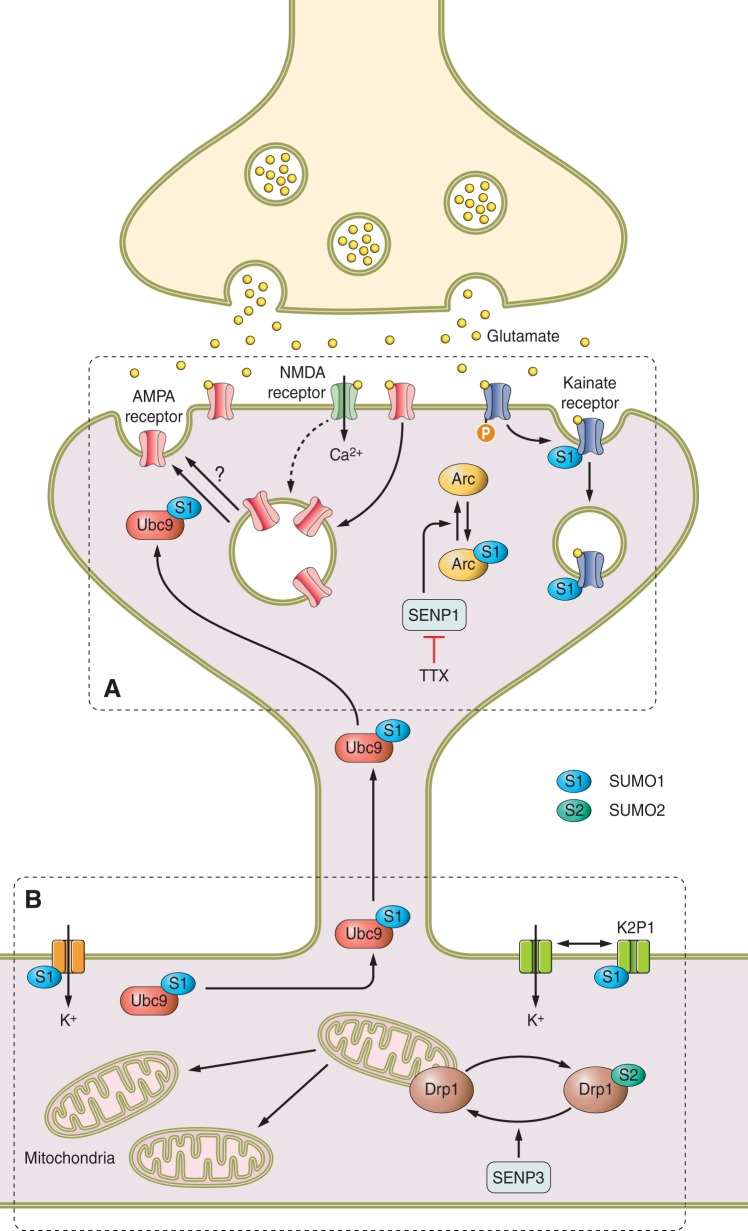
FIGURE 7.
SUMOylation at the postsynapse. SUMOylation regulates postsynaptic structure and function through modification of multiple substrates. A: agonist activation of the kainate receptor (KAR) leads to PKC-mediated phosphorylation and SUMOylation of the GluK2 receptor subunit, ultimately resulting in KAR endocytosis. Although not direct targets themselves, SUMOylation also regulates the trafficking of AMPA receptors. Induction of LTP with the NMDAR coagonist glycine recruits Ubc9 and SUMO to the postsynapse, and SUMOylation is essential for AMPAR insertion into the postsynaptic membrane. Suppression of synaptic activity by TTX blockade of voltage-gated sodium channels causes increased AMPAR insertion and synaptic upscaling. This treatment also causes the loss of the SUMO protease SENP1, promoting SUMOylation of the synaptic protein Arc, which in turn reduces AMPAR endocytosis, resulting in increased AMPAR surface expression. B: SUMOylation modulates neuronal excitability through modification of the potassium channels K2P1 and Kv1.5, and potentially regulates the ability of synapses to meet energy demands through modification of the mitochondrial GTPase Drp1, which controls mitochondrial number.