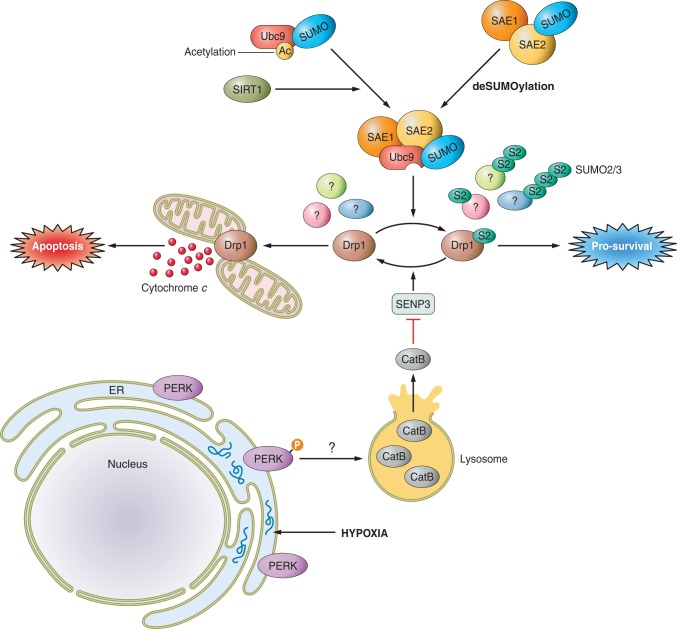
FIGURE 8.
SUMOylation and ischemia. Hypoxia induces the accumulation of unfolded proteins in the ER, resulting in ER stress, which is sensed by the ER-resident kinase PERK. Activation of PERK, through an unknown mechanism, results in lysosomal rupture and release of the protease cathepsin B (CatB) into the cytosol, which mediates degradation of the SUMO2/3-specific SUMO protease SENP3. Loss of SENP3 leads to increased SUMOylation of multiple SUMO2/3 substrates, including the mitochondrial GTPase Drp1. Drp1 is a crucial mediator of mitochondrial fission and pro-apoptotic cytochrome c release. Enhanced SUMOylation of Drp1 during ischemia favors its distribution to the cytosol, where it is unable to stimulate cytochrome c release, promoting cell survival. In addition to stimulating degradation of SENP3, hypoxia also promotes SUMOylation by inducing SIRT1-mediated deacetylation of Ubc9, which enhances its activity against NDSM-containing substrates, and deSUMOylation of the E1 subunit SAE2, which promotes its activity.