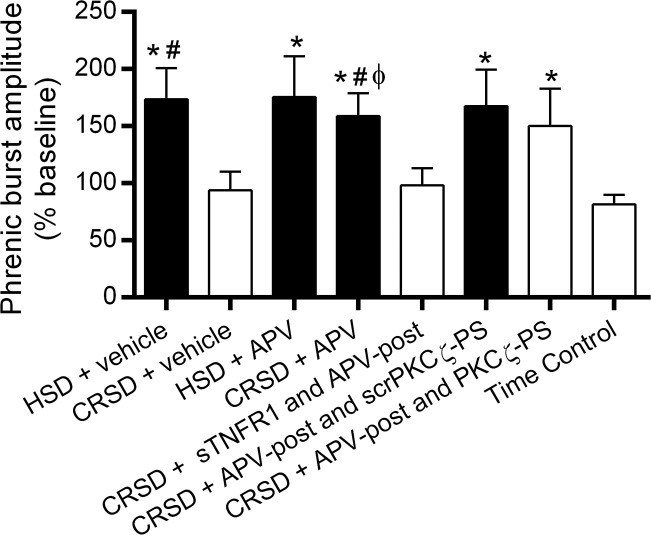
Fig. 5.
Long-lasting iPMF is associated with an enhanced phrenic burst amplitude response to hypercapnia. Average change in phrenic burst amplitude during hypercapnia (% baseline) following neural apnea or an equivalent duration in time controls. Shaded bars indicate significant iPMF at 60 min. As expected, all rat groups exhibited a significant increase in phrenic burst amplitude in hypercapnia. HSD rats receiving intrathecal vehicle or APV prior to neural apnea expressed a significantly enhanced phrenic burst amplitude response to hypercapnia compared with time controls exposed to hypercapnia. By contrast, phrenic burst amplitude during hypercapnia in CRSD rats receiving vehicle was not significantly increased compared with the hypercapnic response in time controls. However, CRSD rats receiving intrathecal APV or intrathecal APV and scrPKCζ-PS expressed a significantly increased phrenic burst amplitude response during hypercapnia relative to the response in time controls and CRSD rats receiving vehicle, suggesting that an enhanced hypercapnic response following reduced respiratory neural activity is enabled by NMDAR inhibition. Intrathecal sTNFR1 impaired the enhanced phrenic hypercapnic response in CRSD rats with NMDAR inhibition, suggesting that spinal TNF-α is necessary for the enhanced phrenic response to hypercapnia. By contrast, CRSD rats receiving APV-post and PKCζ-PS continued to express an increased phrenic hypercapnic response, suggesting that aPKC activity is not necessary for the enhanced phrenic response to hypercapnia following reduced respiratory neural activity (see discussion). Values are means ± SE. *Significantly different than time controls; #significantly different than CRSD + vehicle; ϕsignificantly different than CRSD + sTNFR1 and APV-post; P < 0.05.