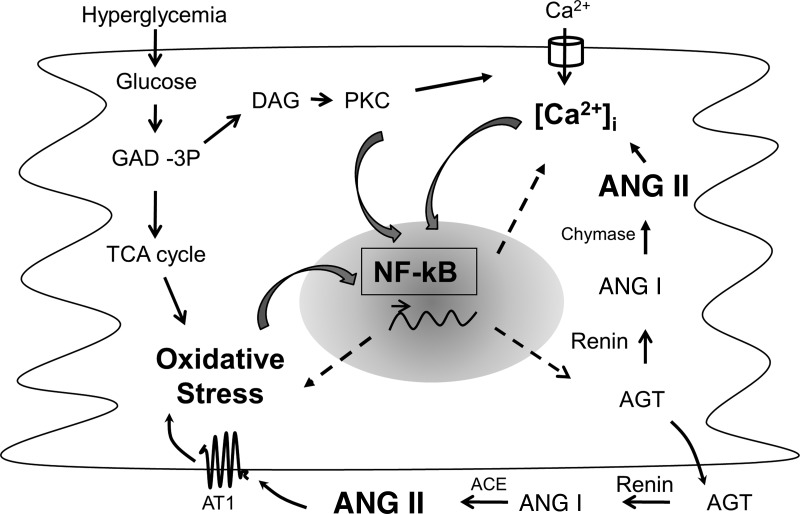
Fig. 8.
Schematic representation of the putative cross-talk between NF-κB, oxidative stress, intracellular Ca2+, and the RAS in cardiac myocytes exposed to hyperglycemia. Hyperglycemia increases oxidative stress as a byproduct of glucose metabolism and intracellular Ca2+ levels through intermediates of the glycolytic pathway. Oxidative stress and intracellular Ca2+ have been shown to activate NF-κB signaling. NF-κB controls the transcription of genes of RAS components, enzymes that modulate oxidative stress (e.g., NADPH oxidase), and Ca2+-handling proteins. ANG II, the bioactive peptide of the RAS, in turn increases oxidative stress and intracellular Ca2+ levels and activates NF-κB signaling. Thus, NF-κB appears to be the nodal point for the convergence of major cellular events that contribute to cardiac dysfunction in diabetes. This study provides evidence that lack of NF-κB activation prevents diabetic cardiomyopathy. GAD-3P, glyceraldehyde 3-phosphate; DAG, diacylglycerol; TCA cycle, tricarbocylic acid cycle; AT1, ANG II type 1 receptor; ACE, angiotensin-converting enzyme; [Ca2+]i, intracellular Ca2+ concentration.