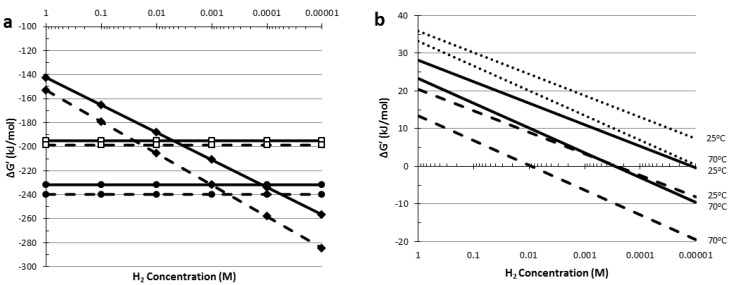
Figure 6.
Effect of the H2 concentration on the Gibbs energy change of reactions involved in H2 formation: (a) ΔG' of the complete oxidation of 1 mol of glucose to acetate (closed diamonds), lactate (open squares) and ethanol (closed circles) at 25 °C (solid lines) and 70 °C (dashed lines); (b) ΔG' of H2 formation from NADH (dotted line), reduced ferredoxin (dashed line) and via the bifurcating system (50% NADH and 50% Fdred) (solid line) at 25 °C and 70 °C. Values were calculated from data presented in ([80,81,82,83,84]).