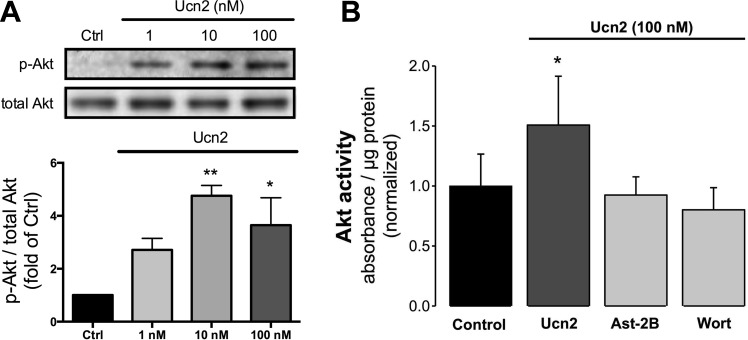
Fig. 2.
Ucn2 elicits a concentration-dependent increase in phosphorylation of Akt and increases Akt kinase activity via activation of corticotropin-releasing factor 2 receptors (CRFR2). A: original immunoblots (top) and average data (bottom) of changes in phosphorylation of Akt at Ser473 induced by 1, 10, and 100 nM Ucn2 (n = 3). Average data of the ratio of phosphorylated to total Akt were normalized to the untreated Ctrl. B: Ucn2-induced increase in Akt activity. Average data of Ucn2 (100 nM)-induced changes in Akt kinase activity (n = 5) in the absence (Ucn2) and presence of astressin-2B (Ast-2B; 1 μM) or wortmannin (Wort; 0.3 μM). Absorbance at 450 nm was normalized to protein concentration. *P < 0.05 and **P < 0.01 vs. Ctrl.