Figure 6.
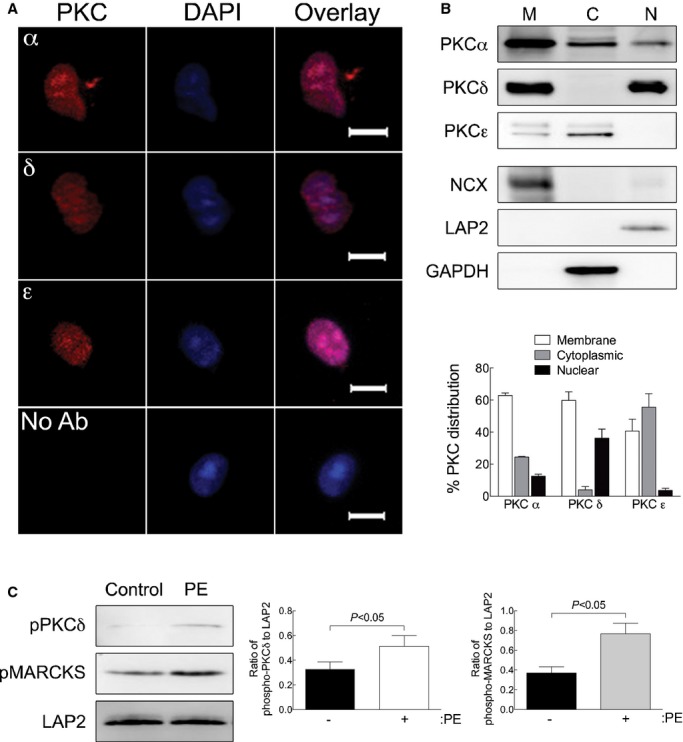
α1‐ARs activate PKC in nuclei isolated from adult cardiac myocytes. A, Nuclei were isolated from adult cardiac myocytes and stained with antibodies to the PKCα, δ, or ε isoforms followed by DAPI counterstain (scale bar=5 μm). B, PKCα, δ, or ε isoform distribution in membrane (M), cytosolic (C), and nuclear (N) was measured by Western blot analyses. Fraction purity was verified by Western blots for NCX (membrane), GAPDH (cytosolic), and LAP2 (nuclear). Relative distribution of the PKC isozymes is presented as mean±SEM for 3 separate nuclear preparations. C, PKC activation was measured in isolated nuclei treated with PE (20 μmol/L, 2 μmol/L of timolol) by phosphorylation of PKCδ at Thr505. PKC activity was measured by phosphorylation of the PKC substrate, MARCKS (pMARCKS). LAP2, a nuclear marker, was used as a loading control. Quantitation of PKCδ phosphorylation at Thr505 and MARCKS phosphorylation is presented as mean±SEM from 3 separate nuclear preparations. Data were analyzed by Student's t test, and significant comparisons are indicated as P<0.05. GAPDH indicates glyceraldehyde 3‐phosphate dehydrogenase; LAP2, lamin‐associated protein 2; NCX, Na+/Ca2+ exchanger; PE, phenylephrine; PKC, protein kinase C; pMARCKS, phospho‐myristoylated alanine‐rich C kinase substrate; α1‐ARs, α1‐adrenergic receptors.
