Figure 1.
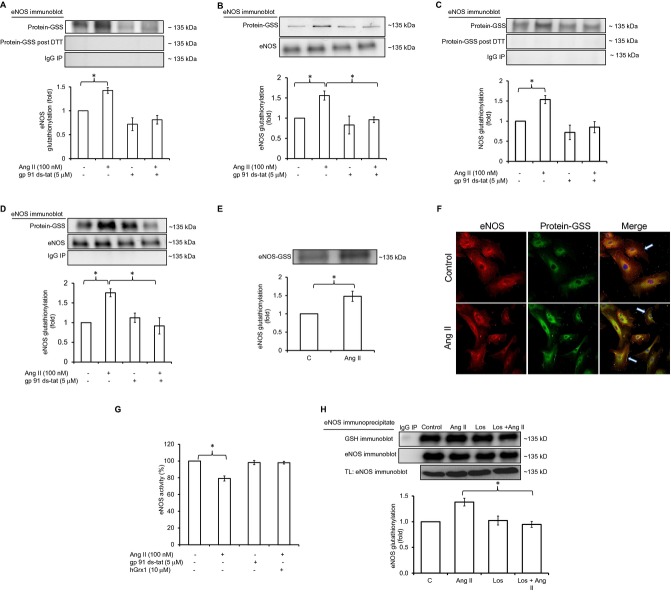
Ang II increases eNOS glutathionylation and inhibits its activity through glutathionylation in an NADPH oxidase‐dependent manner. A, eNOS immunoblotting of GSH or IgG immunoprecipitate (IP) from HUVEC lysate with or without DTT (n=5). B, eNOS immunoblotting of total lysate and streptavidin precipitate (protein‐GSS) of HUVECs loaded with biotinylated GSH before Ang II exposure (n=5). C, eNOS immunoblotting of GSH IP from rabbit aorta (n=5). D, GSH immunoblotting of eNOS IP from rabbit aorta (n=5). E, eNOS immunoblotting of GSH IP from human arterial segments (n=4). F, Immunostaining of eNOS (first column, red fluorescence) and glutathionylation (second column, green fluorescence) in control HUVECs and cells treated with Ang II. The merged eNOS/glutathionylation image demonstrates in situ colocalization of glutathionylation and eNOS immunostaining (third column, yellow fluorescence, white arrows point to colocalization at membrane). Nuclei were stained with DAPI (blue). G, eNOS activity in HUVECs±Ang II, measured by the conversion of l‐[14C]arginine to l‐[14C]citrulline in cell lysates. Preincubation of cells with gp 91ds‐tat (5 μmol/L); or cell lysate with hGrx1 (10 μg/mL) is as indicated (n=6). H, eNOS glutathionylation in HUVECs preincubated with losartan (Los). Statistical comparison was made between the Ang II and Ang II+Los groups (n=4). Results are shown as means±SEM. Statistical significance (P<0.05) is indicated by asterisk. DTT indicates dithiotreitol; eNOS, endothelial nitric oxide synthase; GSH, glutathione; HUVECs, human umbilical vein endothelial cells.