Figure 5.
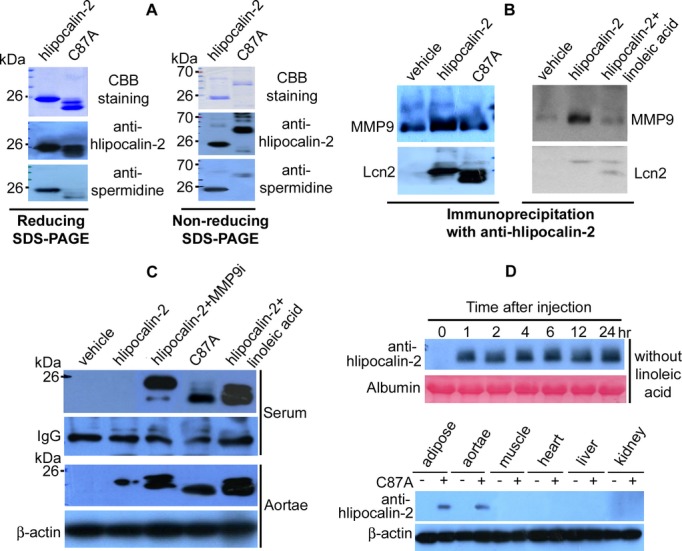
Reduced polyamination of human lipocalin‐2 mutant C87A. A, Recombinant hlipocalin‐2 and C87A mutant (3 μg) were separated by reducing (left panel) or nonreducing (right panel) SDS‐PAGE and staining with Coomassie Brilliant Blue (CBB). After transferring to PVDF membrane, Western blotting was performed to detect spermidine. B, Coimmunoprecipitation was performed using 30 μL of serum from Lcn2‐KO mice treated with vehicle, hlipocalin‐2, C87A, or hlipocalin‐2 plus linoleic acid (LA). Immune complexes were separated by SDS‐PAGE and subjected to Western blotting using antibodies recognizing MMP9 or hlipocalin (Lcn2). C, Wild‐type hlipocalin‐2 or C87A was given to Lcn2‐KO mice under standard chow. Before administration of hlipocalin‐2, mice were treated with or without MMP9 Inhibitor I (MMP9i; 2 μg/mouse, tail vein injection) or LA (3 mg/mouse, intraperitoneal injection). Six hours after treatment, sera and aortae were collected for Western blotting to evaluate hlipocalin‐2 levels. Nonspecific serum IgG and β‐actin was probed as the loading controls, respectively. D, Lcn2‐KO mice under standard chow were treated with C87A, without combined administration of LA. At different time points after injection, serum was collected from tail vein and subjected to Western blotting analysis of circulating C87A using hlipocalin‐2 antibody (upper panel). At the end of treatment, tissues were also collected for analyzing the accumulated C87A protein (lower panel). IgG indicates immunoglobulin G; Lcn2‐KO, lipocalin‐2 knockout; MMP9, matrix metalloproteinase 9.
