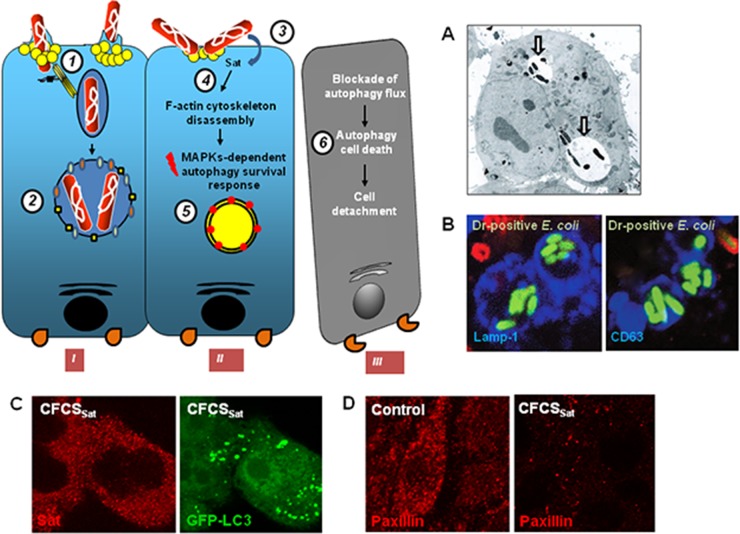
FIG 6.
Cellular events observed in cervical epithelial HeLa cells infected with uropathogenic Afa/Dr DAEC or subjected to Sat toxin treatment. The drawing on the left summarizes the observed cellular events. Internalization of bacteria occurs in nonpolarized epithelial cells via the recognition of membrane-associated hDAF, or hCEACAM1, hCEA, and hCEACAM6 by a mechanism involving lipid rafts and dynamic microtubules (1). Internalized Afa/Dr DAEC cells have survived within large vacuoles, which seems to result from the fusion of early vacuoles, each containing one bacterium formed during the initial step of internalization, that tested positive for early and late endosome markers but not for the autophagy LC3 marker (2). The secreted and cell internalized Sat toxin (3) promotes a dramatic loss of F-actin stress fibers (4), and in turn the intoxicated cell engage an autophagy cell survival response (5). The massive appearance of autophagosomes is followed by the blockade of autophagy flux, leading to the lack of maturation of autophagosomes into autophagolysosomes (5). As the result of autophagic cell death, the cells lose the focal adhesion contacts and become detached from the substratum (6). (A) Transmission electron micrograph showing vacuoles containing internalized Afa-III adhesin-positive E. coli in HeLa cells. Arrows indicate the vacuole-containing internalized bacteria. (Reprinted from reference 332 with the permission of the publisher. Copyright 2003 Blackwell Publishing Ltd.) (B) High-magnification micrographs show the observation by CLSM of Lamp1 or CD63 immunolabeling (blue) in membranes of vacuoles containing the internalized Dr adhesin-positive E. coli in infected HeLa cells (green). Red, extracellular adhering bacteria. (Reprinted from reference 189.) (C) High-magnification micrographs show the observation by CLSM of Sat immunolabeling present in the cytoplasm of cells treated with cell-free culture supernatant of AAEC185pSatIH11128 containing the secreted Sat toxin (CFCSSat) (red) and the appearance of green fluorescent protein (GFP)-LC3 autophagic vacuoles in CFCSSat-treated cells (green). (Reprinted from reference 374 with the permission of the publisher. Copyright 2011 Blackwell Publishing Ltd.) (D) High-magnification micrographs show the observation by CLSM of paxillin immunolabeling (red). Note the disappearance of paxillin in in CFCSSat-treated cells. (Reprinted from reference 374 with the permission of the publisher. Copyright 2011 Blackwell Publishing Ltd.)