FIG 9.
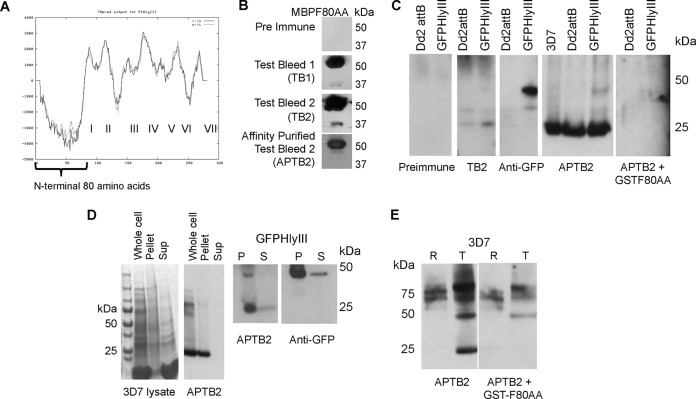
Generation of rabbit polyclonal antiserum against the N terminus (first 80 amino acids) of PfHly III, affinity purification, and detection of PfHly III in asexual blood stages. (A) TMpred-generated prediction of transmembrane regions I to VII in PfHly III, with the first N-terminal 80 amino acids indicated with no transmembrane domains. (B) Test bleed antiserum recognizes recombinant MBP-tagged PfHly III N-terminal 80-amino-acid peptide (MBPF80AA, 53 kDa) compared to the preimmune serum. (C) Affinity-purified test bleed 2 (APTB2) recognizes a unique band of less than 30 kDa in three strains of P. falciparum: 3D7, Dd2attB, and Dd2attB transfected with PfHly III-GFP; anti-GFP and APTB2 both recognize a unique higher-molecular-weight product in the PfHly III-GFP-transfected strain. (D) A 25-kDa product is located in the pellet (P) and soluble (S) fractions of both 3D7 and PfHly III-GFP-transfected strains of P. falciparum for native PfHly III. The 25- and 50-kDa products in the PfHly III-GFP-expressing parasites are also found in the pellet and supernatant fractions. Approximately 10:1 amounts of protein were loaded for the pellet/supernatant ratio seen in the Western blot. (E) Comparing synchronized rings (R) and trophozoite (T) stages normalized by protein content, the native 25-kDa product was primarily observed in trophozoites, with signal from the native protein removed by competition with recombinant GSTF80AA antigen.