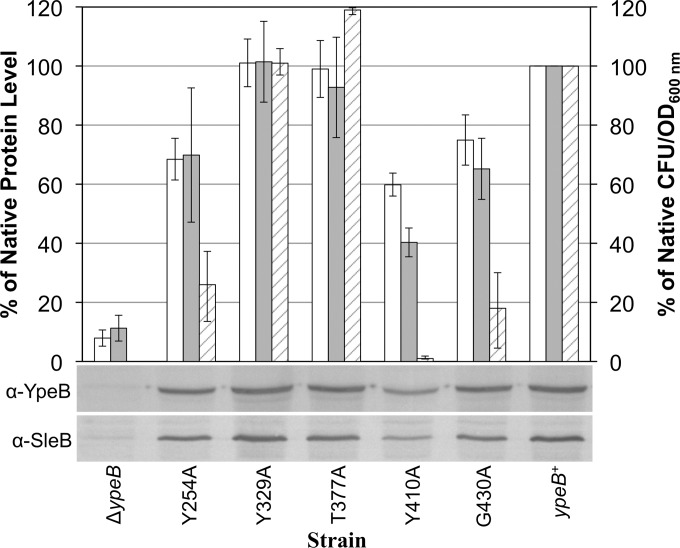
FIG 5.
Protein stability and plating efficiency in YpeB point mutant spores. Dried, dormant ΔypeB, YpeBY254A, YpeBY329A, YpeBT377A, YpeBY410A, YpeBG430A, and ypeB complementation (ypeB+) spores were broken, and extracted proteins were analyzed by Western blotting with anti-SleB (α-SleB) or anti-YpeB (α-YpeB) antibodies. Band intensities were quantified and are depicted as the levels of YpeB (open bars) and SleB (shaded bars) present in spores, relative to the native protein levels found in ypeB+ spores, which were set at 100%. Spores of the same strains were heat activated, serially diluted, and plated on BHI medium. Following incubation, colonies were counted, and the percentages of native CFU/OD values (striped bars) were determined by comparison to the CFU/OD value for ypeB+ spores, which was set at 100%. Plating efficiency and Western blot quantification data shown are averages from three independent spore preparations; error bars represent the standard deviations. The Western blots below the graph show representative results from one of the replicates.