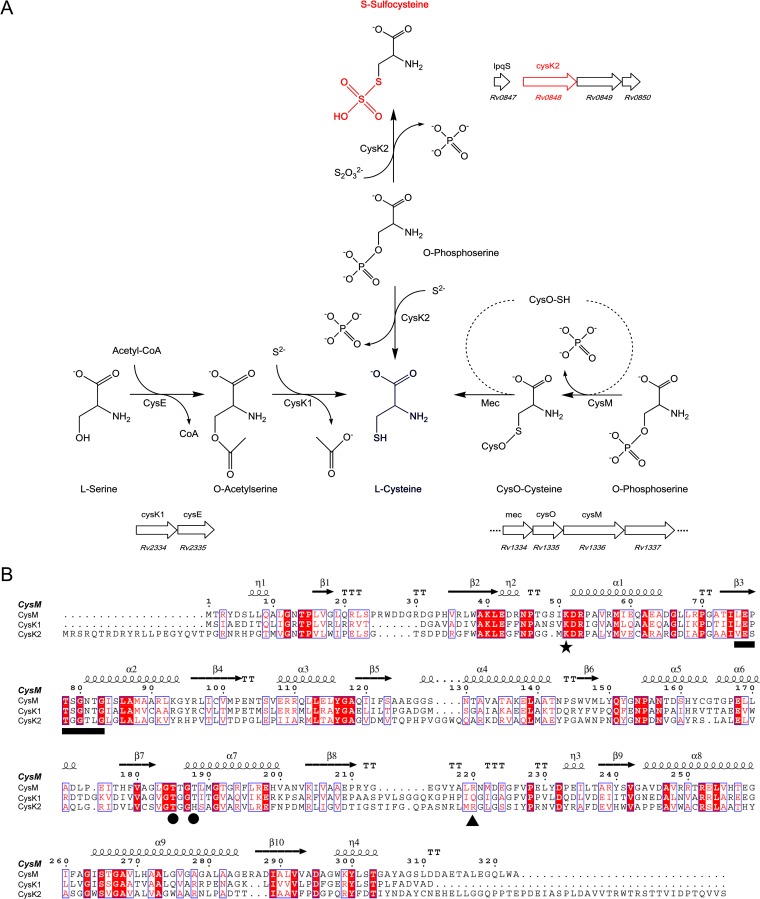
FIG 1.
Comparison of cysteine synthases and CysK2 in M. tuberculosis. (A) l-Cysteine production in M. tuberculosis through three pathways. The classical pathway (left), present in most eubacteria and plants, uses CysK1Mtb for the conversion of O-acetyl-l-serine (OAS) and hydrogen sulfide to l-cysteine. The alternative pathway (right) in M. tuberculosis is based on CysMMtb for l-cysteine formation from O-phospho-l-serine (OPS) and thiocarboxylated CysO. The third pathway (middle), based on CysK2, uses OPS and hydrogen sulfide or thiosulfate for cysteine or S-sulfocysteine production, respectively. The organization of the operons including the three cysteine synthases (CysK1, CysM, and CysK2) in the M. tuberculosis H37Rv genome is also shown (http://tbdb.org). (B) Structure-based sequence alignment of CysK1Mtb, CysMMtb, and CysK2 using CysMMtb as the reference structure, with the secondary structure elements displayed above the alignments. The position of the invariant lysine residue that covalently binds the cofactor *), residues binding the cofactor phosphate group (●), the motif involved in H-bonding interactions with the substrate carboxylate (black horizontal bar), and the position of the arginine residue responsible for OPS specificity in CysMMtb (▲) are indicated. The image in panel B was created with ESPript (35).