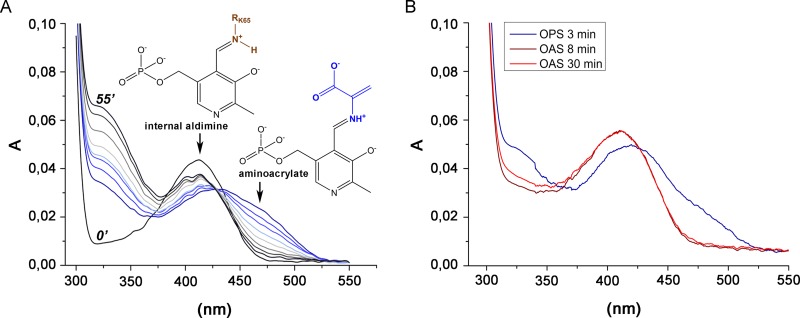
FIG 3.
UV-visible spectra of holo-CysK2 reactivity and enzyme-substrate complexes. (A) The UV-visible spectrum of CysK2 shows a maximum at 412 nm (black line), typical of the internal aldimine. Addition of 2 mM OPS results in fast enzyme-aminoacrylate intermediate formation indicated by an absorption increase at 330 nm and the shift of the PLP peak from 412 nm to 460 to 470 nm. Spontaneous decay of the aminoacrylate intermediate is demonstrated by the set of spectra recorded after 3, 6, 9, 14, 20, 30, 40, and 55 min following addition of OPS (blue-gray). Spectra at time points 0 and 55 min are labeled. (B) Comparison of the UV-visible spectra of CysK2 with OPS or OAS as the substrate. OPS addition results in spectral changes consistent with the formation of the enzyme-aminoacrylate intermediate (blue trace). The addition of 2 mM OAS does not indicate enzyme-aminoacrylate intermediate formation after 3 min or 30 min of incubation (red and orange traces).