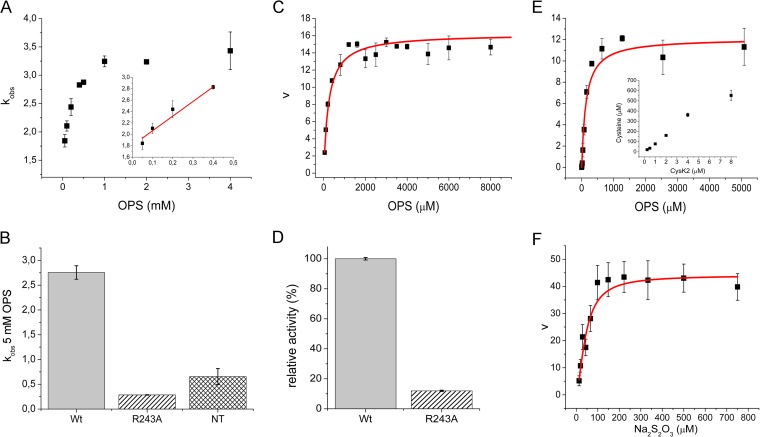
FIG 5.
Kinetic characterization of CysK2, CysK2R243A mutant, and CysK2NT. (A) First half-reaction parameters investigated by stopped-flow spectrophotometry. Pseudo-first-order rate constants (kobs) at different OPS concentrations. The linear fit used to derive the second-order rate constant (kmax/Ks) is shown in the inset. (B) Comparison of kobs at 5 mM OPS between wild-type CysK2 (wt), CysK2R243A (R243A), and CysK2NT (NT) constructs. (C) Michaelis-Menten kinetics of CysK2 based on phosphate release using the malachite green calorimetric assay. (D) Relative activity of wild-type CysK2 compared to CysK2R243A based on inorganic phosphate release. (E) Michaelis-Menten kinetics of CysK2 in the presence of sulfide as a sulfur donor substrate. The inset shows the dependence of l-cysteine production on enzyme concentration. (F) Michaelis-Menten kinetics of CysK2 in the presence of thiosulfate as an S donor substrate.