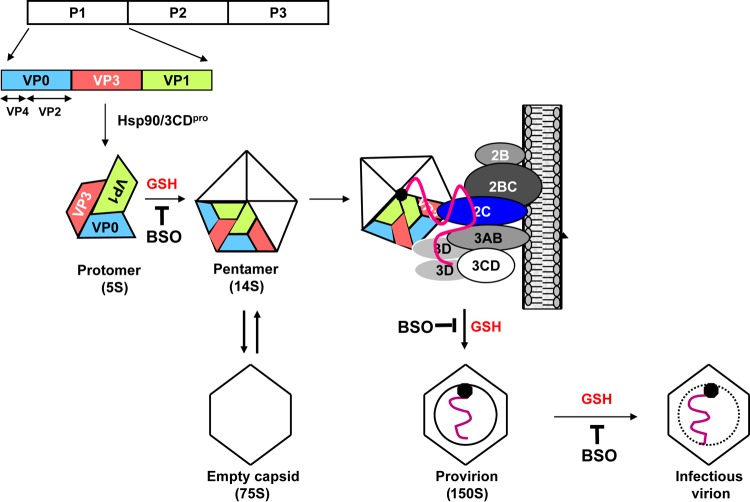
FIG 5.
Model for enterovirus morphogenesis. After the newly made capsid precursor P1 is released from the polyprotein, it interacts with the chaperone Hsp90 to assume a conformation competent for cleavage by 3CDpro. After proteolytic processing, a protomer is formed spontaneously, consisting of one copy each of VP0, VP1, and VP3. In the presence of GSH, five protomers assemble to generate a pentamer, which, by interactions between VP3 and 2CATPase or between VP1 and 2CATPase, is recruited to the replication complex to associate with the newly made VPg-linked plus-strand viral RNA. Subsequently, 12 pentamers condense around the RNA to produce a noninfectious provirion. The last step is the maturation cleavage of VP0, which is autocatalytic and RNA dependent and yields a stable particle containing 60 copies of VP1 and VP3, 59 copies of VP2 and VP4, and 1 copy of VP0. The steps at which GSH is required, as shown by inhibition with BSO, are marked.