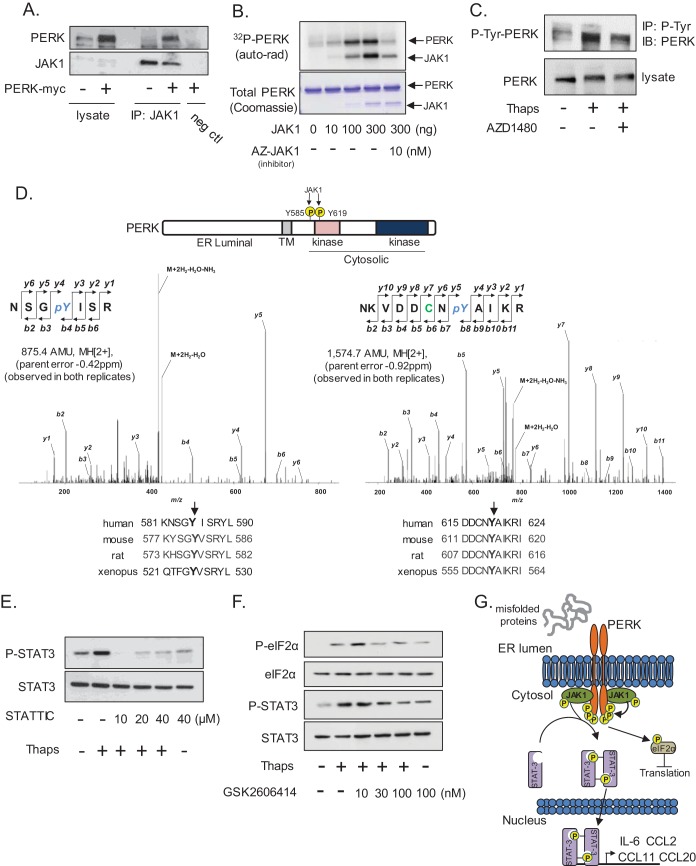
FIG 8.
JAK1 interacts with and phosphorylates PERK. (A) Astrocytes were transfected with empty vector (−) or PERK-myc (+) followed by immunoprecipitation of JAK1 and immunoblotting for PERK and JAK1. Antibody was omitted in the negative control (neg ctl). (B) Recombinant PERK (2 μg cytoplasmic domain amino acids [aa] 536 to 1116) was used as a substrate in an in vitro kinase assay with increasing amounts of recombinant active JAK1. The JAK1 inhibitor AZ-JAK1 (10 nM) was included in the kinase reaction where indicated. (C) Astrocytes were treated with Thaps (1 μM, 1 h) in the absence or presence of AZD1480 (1 μM). Total phosphotyrosine was immunoprecipitated and immunoblotted for PERK. (D) An in vitro kinase assay was performed as described for panel B with nonradioactive ATP, and PERK was analyzed by LC-ESI-MS/MS. The identified phosphorylation sites, the respective MS/MS spectra, and the homology of each site are shown. (E) Astrocytes were treated with Thaps (1 μM, 1 h) in the absence or presence of the indicated concentrations of STATTIC. (F) Astrocytes were treated with Thaps (1 μM, 2 h) in the absence or presence of increasing concentrations of the PERK kinase inhibitor GSK2606414, followed by immunoblotting. (G) Potential signaling model in which JAK1 phosphorylates PERK and mediates the ER stress-induced activation of STAT3.