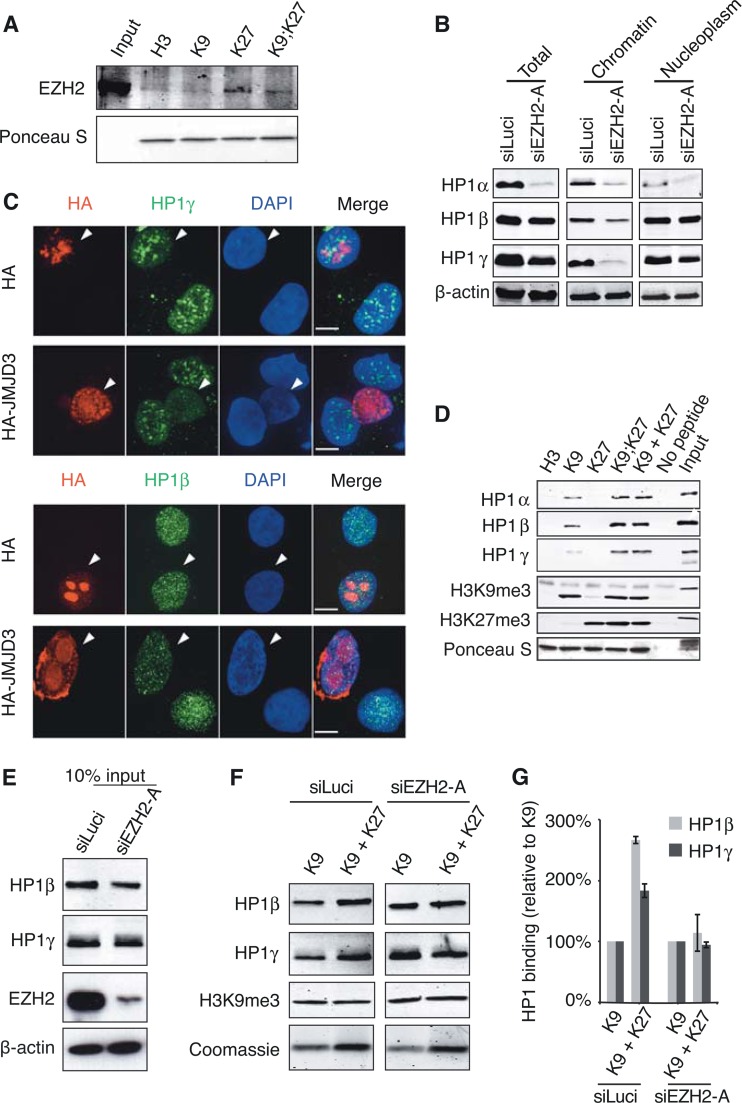
FIG 7.
EZH2 is required for increased binding of human cell extract-derived HP1β/γ to H3 histone tails with both K9me3 and K27me3 marks. (A) EZH2 binding is detected on K27me3-containing H3 tails. HT1080 cell lysates were incubated in the presence of immobilized biotinylated H3 peptides modified as indicated, and bound EZH2 was detected by Western blotting. The total amount of H3 peptides was monitored with Ponceau S. (B) Residual HP1α/β/γ levels upon EZH2 depletion. HT1080 cells were transfected with either siLuci or siEZH2-A and collected 72 h after transfection. Total cell extracts and chromatin and nucleoplasm fractions were analyzed by Western blotting using the indicated antibodies. (C) Immunofluorescence analyses of HP1β and HP1γ (green) in HT1080 cells transiently transfected with HA-JMJD3 construct and performed as described in legend to Fig. 4A to C. (D) Pulldown experiments with HT1080 cell lysates and biotinylated H3 tail peptides (H3, unmodified; K9, H3K9me3; K27, H3K27me3; K9;K27, H3K9me3K27me3; K9 + K27, equimolar mix of H3K9me3 and H3K27me3). (E to G) Binding of HP1β and γ to H3 tails with K9me3 and K27me3 is reduced in the absence of EZH2. (E) Western blot of cell extracts after HT1080 treatment with siLuci or siEZH2-A used as inputs for pulldown experiment shown in panel F. (F) H3K9me3 or a mixture of H3K9me3 and H3K27me3 peptides was used for the pulldown experiment. Bound HP1β/γ and H3K9me3 mark levels were monitored by Western blotting. Loading of H3 peptides was monitored by Coomassie blue staining. (G) Quantification of data from panel F by normalizing to H3K9me3 binding.