Abstract
The host inflammatory response contributes to the tissue damage that occurs during amebic colitis, with tumor necrosis factor alpha (TNF-α) being a key mediator of the gut inflammation observed. Mammalian macrophage migration inhibitory factor (MIF) is a proinflammatory cytokine that plays an important role in the exacerbation of a wide range of inflammatory diseases, including colitis. We identified a MIF gene homolog in the Entamoeba histolytica genome, raising the question of whether E. histolytica MIF (EhMIF) has proinflammatory activity similar to that of mammalian MIF. In this report, we describe the first functional characterization of EhMIF. Antibodies were prepared against recombinantly expressed EhMIF and used to demonstrate that EhMIF is expressed as a 12-kDa protein localized to the cytoplasm of trophozoites. In a manner similar to that of mammalian MIF, EhMIF interacted with the MIF receptor CD74 and bound to macrophages. EhMIF induced interleukin-6 (IL-6) production. In addition, EhMIF enhanced TNF-α secretion by amplifying TNF-α production by lipopolysaccharide (LPS)-stimulated macrophages and by inhibiting the glucocorticoid-mediated suppression of TNF-α secretion. EhMIF was expressed during human infection, as evidenced by the presence of anti-EhMIF antibodies in the sera of children living in an area where E. histolytica infection is endemic. Anti-EhMIF antibodies did not cross-react with human MIF. The ability of EhMIF to modulate host macrophage function may promote an exaggerated proinflammatory immune response and contribute to the tissue damage seen in amebic colitis.
INTRODUCTION
Entamoeba histolytica is the protozoan parasite that causes amebic colitis. Diarrheal disease is second only to pneumonia as a leading cause of death globally in children under the age of 5 years, and intestinal amebiasis is one of the leading causes of severe diarrhea in the developing world (1, 2). For example, in a cohort of Bangladeshi infants, E. histolytica was present in 20% of all diarrheal episodes (3). Infection with E. histolytica trophozoites results in marked mucosal inflammation. Along with E. histolytica cytolytic factors, the host inflammatory response contributes to the tissue destruction seen in amebic colitis (4). Tumor necrosis factor alpha (TNF-α) is a proinflammatory cytokine that plays a central role in intestinal inflammation, including the gut inflammation seen in amebic colitis. More TNF-α production was shown to correlate with E. histolytica diarrhea in children, and blocking of TNF-α with monoclonal antibodies reduced inflammation and intestinal damage from amebic infections in mice (5, 6). TNF-α was also recently shown to mediate the tissue destruction seen in amebic liver abscesses (7).
Mammalian macrophage migration inhibitory factor (MIF) is a pleiotropic cytokine with mitogenic and proinflammatory functions (8). Proinflammatory functions include the following: (i) MIF induces the secretion of inflammatory mediators, such as interleukin-6 (IL-6); (ii) MIF enhances TNF-α production by lipopolysaccharide (LPS)-stimulated immune cells; and (iii) MIF can counterregulate the anti-inflammatory activities of glucocorticoids (9–17). Many of the inflammatory effects of MIF are initiated via direct binding to its cell surface receptor, CD74 (18). Several studies have implicated a key role for MIF in colitis. In one study of patients with inflammatory bowel disease (IBD), a condition characterized by inflammation of the colon, patients with IBD were found to have significantly higher concentrations of MIF in plasma (17). Furthermore, there is genetic evidence to support the role of MIF in colitis. The MIF −173 G/C single nucleotide polymorphism is associated with elevated MIF expression and, in turn, increased susceptibility to IBD (19, 20). Additionally, MIF knockout mice are protected from dextran-induced colitis, while antibodies targeting MIF prevent experimental colitis (21, 22).
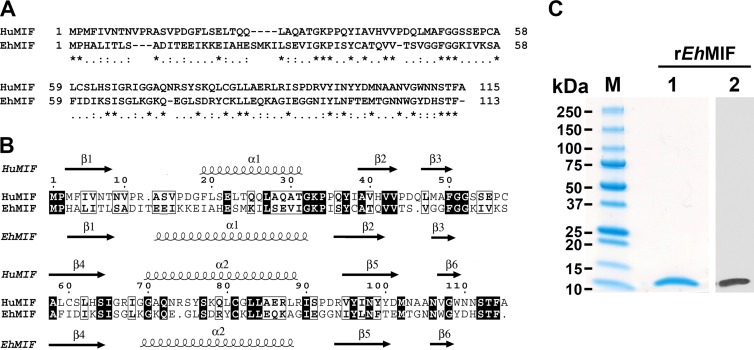
MIF homologs from several pathogenic protozoans have been characterized (23–28). We identified a MIF homolog in the E. histolytica genome (EHI_092370). The E. histolytica MIF (EhMIF) gene has a coding sequence consisting of 342 bp, with no intron and a GC content of 31%. The gene encodes a protein with a predicted molecular mass of 12 kDa. Microarray studies showed that there is at least an 8-fold increase in EhMIF expression from the dormant cyst stage to the invasive and proliferative trophozoite stage (29, 30). Similar to other protozoan MIF homologs, EhMIF has a relatively low identity (28%) to human MIF but appears to be structurally conserved (Fig. 1). Protozoan MIF homologs have demonstrated biological activity similar to that of mammalian MIF. For example, Plasmodium MIF was shown to enhance TNF-α secretion by immune cells after stimulation with LPS (11, 16). The presence of a MIF homolog in E. histolytica implies a potential mechanism that may contribute to the proinflammatory host response that occurs during infection.
FIG 1.
Characterization of EhMIF. (A) Pairwise amino acid sequence alignment of human MIF (HuMIF) and Entamoeba histolytica MIF (EhMIF). Sequence alignment was performed using Needle (EMBOSS). Symbols: asterisks, identical residues; colons, conserved residues; periods, semiconserved residues. (B) Secondary structure homology between HuMIF and EhMIF. The secondary structure elements of HuMIF and EhMIF are shown above and below the alignment, respectively. The figure was generated with ClustalW2 and ESPript. (C) Purified recombinant EhMIF (2 μg) in an SDS-PAGE gel stained with Coomassie Blue (lane 1), with an estimated molecular mass of 12 kDa, and immunoblot analysis of purified recombinant EhMIF (25 ng), using anti-penta-His–HRP conjugate (lane 2).
The role of the EhMIF homolog in E. histolytica-induced gut inflammation has not yet been characterized. We generated recombinant EhMIF and examined its ability to modulate host macrophage function. We also investigated the cellular localization of EhMIF and whether anti-EhMIF antibodies were present in the sera of children living in an area where E. histolytica infection is endemic.
MATERIALS AND METHODS
Expression and purification of recombinant EhMIF.
The EhMIF gene was codon optimized for Escherichia coli expression and cloned into the pJexpress414 vector (DNA2.0). The expression plasmid was transformed into E. coli BL21(DE3) competent cells. Expression of EhMIF with a C-terminal polyhistidine tag was induced at an optical density at 600 nm (OD600) of 0.6 by use of isopropyl-β-d-thiogalactopyranoside (IPTG) for 3 h at 37°C. The pelleted cells were sonicated, and the bacterial extract was purified by Ni-nitrilotriacetic acid (Ni-NTA) chromatography (Qiagen) and dialyzed into phosphate-buffered saline (PBS). Protein concentrations were determined by Bradford assay (Thermo Scientific). Sample purity was analyzed by SDS-PAGE. Protein expression was also confirmed by immunoblotting, using an anti-penta-His–horseradish peroxidase (HRP) conjugate (Qiagen). Triton X-114 and polymyxin B-agarose (Sigma) were used during wash and purification procedures for removal of endotoxin. The resulting proteins contained <1 pg LPS/μg protein as quantified by the Limulus amebocyte lysate (LAL) assay (Thermo Scientific). His-tagged mouse MIF (MMIF) and glutathione S-transferase (GST) were purified, and endotoxin removed, under the same conditions as those for recombinant EhMIF.
GST pulldown assay.
GST pulldown assays were carried out as previously described (31). Briefly, the mouse CD74 ectodomain cDNA was cloned into the pGEX-6P-1 GST expression vector (GE Healthcare). The empty pGEX-6P-1 vector (GST-only control) and the GST-CD74 expression plasmid were transformed into BL21(DE3) competent cells. Protein expression was induced at an OD600 of 0.6 by the addition of IPTG. Approximately 0.25 μg of GST or GST-CD74 in bacterial lysate was added to glutathione Sepharose beads (GE Healthcare). After washing with PBS, recombinant EhMIF was added to a final concentration of 1 μM in 500 μl binding buffer (50 mM KxHyPO4 [pH 7.2], 50 mM NaCl, 5% glycerol, 0.01% Tween). Mixed fractions were incubated at 4°C for 1 h with rotation. The beads were washed three times with binding buffer and then subjected to SDS-PAGE. EhMIF was detected by immunoblotting with anti-penta-His–HRP conjugate (Qiagen).
Cell binding analyses by flow cytometry.
The cell-binding properties of EhMIF were determined based on a previously described method (32). EhMIF was biotinylated using an EZ-Link sulfo-NHS-LC biotinylation kit (Thermo Scientific) per the manufacturer's instructions. Biotinylated EhMIF (1 μg) was incubated with 2 × 105 murine RAW-Blue macrophages (InvivoGen) for 45 min at 4°C to prevent internalization. In selected experiments, macrophages were incubated with mouse anti-CD74 antibody (Santa Cruz Biotechnology) for 30 min before exposure to EhMIF, or EhMIF was incubated with MMIF at a final dilution of 1:20. After washing with PBS, samples were incubated with streptavidin-Alexa Fluor 647 (Invitrogen) for 30 min. Samples incubated with streptavidin-Alexa Fluor 647 only were used as negative controls. The cells were washed and analyzed by flow cytometry using a FACSCalibur flow cytometer (Becton, Dickinson). Flow cytometry data were analyzed with FlowJo software.
Effects of EhMIF on cytokine production.
RAW-Blue macrophages (106 cells/well in 6-well plates) were stimulated with increasing concentrations of EhMIF (0 to 100 ng/ml) for 8 h. Macrophages were also treated with 25 μg/ml anti-CD74 antibody or control IgG. IL-6 levels in supernatants were measured by enzyme-linked immunosorbent assay (ELISA) (eBioscience) according to the manufacturer's instructions. The effect of EhMIF on TNF-α production was evaluated using a modification of a previously described method (9). RAW-Blue macrophages were incubated for 8 h with EhMIF or EhMIF and LPS (from E. coli O111:B4; Sigma) at 100 ng/ml. TNF-α levels in supernatants were measured by ELISA (eBioscience) according to the manufacturer's instructions. Cytokines secreted by macrophages into culture supernatants were also measured after stimulation with EhMIF preincubated with purified rabbit polyclonal anti-EhMIF IgG or control IgG.
Glucocorticoid override assay.
RAW-Blue macrophages were preincubated for 1 h with 100 nM dexamethasone (Sigma) or with dexamethasone plus EhMIF (100 ng/ml) before the addition of LPS (100 ng/ml). Supernatants from macrophage cultures were collected after 4 h. TNF-α levels in supernatants were measured by ELISA (eBioscience).
Immunoblotting and immunofluorescence assay (IFA).
E. histolytica HM1:IMSS trophozoites in TYI-S33 medium were harvested by centrifugation. The pellet was washed with PBS and sonicated. The lysate was centrifuged at 18,000 × g for 30 min at 4°C, and the supernatant was then subjected to SDS-PAGE. Protein extracts were transferred onto polyvinylidene difluoride membranes (Millipore). The membranes were incubated overnight at 4°C with anti-EhMIF rabbit serum or preimmune rabbit serum followed by peroxidase-conjugated anti-rabbit IgG (Sigma). Peroxidase activity was detected by enhanced chemiluminescence assay (Thermo Scientific).
The localization of EhMIF within trophozoites was determined using fluorescence microscopy. Trophozoites were fixed in 4% formalin for 30 min and then spotted on polylysine-coated slides. The cells were permeabilized with PBS containing 0.2% Triton X-100 for 5 min and blocked with 3% bovine serum albumin (BSA) plus 0.05% Tween 20 in PBS for 1 h at 37°C. IFA was performed using sera containing rabbit polyclonal anti-EhMIF antibodies. Fluorescence staining was achieved with Alexa Fluor 488-conjugated secondary antibodies specific to rabbit IgG (Invitrogen). Nuclei were stained with DAPI (4′,6-diamidino-2-phenylindole). The slides were then mounted with Vectashield mounting medium (Vector Laboratories). Images were captured using an Olympus BX51 fluorescence microscope equipped with an Olympus DP70 digital camera.
ELISA and immunoblot analyses of children's sera.
A total serum IgG ELISA was performed on sera from children. Sera were taken from 160 preschool children (2 to 5 years old) in the area of Mirpur, within Dhaka, Bangladesh, an area where Entamoeba histolytica infection is endemic. The collection of blood samples for research purposes was reviewed and approved by the Institutional Review Board at the University of Virginia and the Ethical Review Committee of the International Center for Diarrheal Disease Research, Bangladesh, Dhaka, Bangladesh. A codon-optimized MIF open reading frame (DNA2.0) was PCR amplified and cloned into the expression vector pGEX-6p-1 (GE Healthcare), using BamHI and SalI sites. Competent E. coli BL21(DE3) cells were transformed with vector alone or with pGEX-6p-1-MIF, and the cells were induced with 1 mM IPTG for 3 h at 37°C. GST alone or GST-MIF was purified using glutathione Sepharose beads (GE Healthcare) per the manufacturer's instructions. Ninety-six-well ELISA plates (Maxisorp; Nunc) were coated overnight with 0.5 μg GST alone or GST-MIF per well. Children's sera, diluted 1:250, followed by peroxidase-conjugated anti-human IgG (1:10,000), were added to the ELISA plates. The optical density (OD) was read at 450 nm. A sample was considered to be positive for anti-EhMIF antibody if the OD reading was >2 standard deviations (SD) above the mean for the GST-only control (33, 34).
Immunoblot analysis was also performed to confirm the presence of anti-EhMIF antibody in children's sera and to evaluate whether anti-EhMIF antibody cross-reacted with recombinant human MIF (HuMIF). Recombinant EhMIF and HuMIF, at 100 ng and 50 ng, were separated by SDS-PAGE. An immunoblot was performed as described above, using 1:100 dilutions of anti-EhMIF antibody-positive and -negative sera.
Data analysis.
All data were analyzed using IBM SPSS 20 and GraphPad Prism 6 software. Results are presented as means ± SD or as individual data points. Comparisons between groups were determined using the independent-sample t test or the paired-sample t test. P values of <0.05 (two-tailed) were considered statistically significant.
RESULTS
EhMIF interacts with the cell surface receptor CD74 and binds to host macrophages.
Mammalian MIF achieves some of its inflammatory effects by binding to the MIF receptor (CD74) through interaction with the CD74 ectodomain (18). This interaction has been shown to be conserved in MIF homologs of Leishmania and Plasmodium spp. (16, 27, 31). We examined the ability of purified recombinant EhMIF (Fig. 1C) to interact with CD74 by performing GST pulldown assays. We expressed and purified the mouse CD74 ectodomain (CD74ec) fused to GST (Fig. 2A and B). As shown in Fig. 2C, CD74ec fused to GST readily pulled down recombinant EhMIF relative to the GST control, demonstrating that EhMIF interacted with the MIF receptor.
FIG 2.
EhMIF interacts with the MIF receptor (CD74) and binds to host macrophages. (A) Schematic representation of the MIF receptor (CD74). IC, TM, and EC, intracellular, transmembrane, and extracellular domains, respectively. Also represented are GST and GST fused to the extracellular domain of CD74 (CD74ec). (B) Purified recombinant proteins used in the GST pulldown assay (arrowheads). Approximately 0.25-μg samples of GST (lane 1) and GST-CD74ec (lane 2) were separated by SDS-PAGE and stained with Coomassie blue. (C) Interaction between EhMIF and CD74ec by GST pulldown assay. GST and GST-CD74ec were mixed with His-tagged EhMIF. One percent of the input (lane 1) and the pull-down material (lanes 2 and 3) were separated by SDS-PAGE, and His-tagged EhMIF was detected by immunoblot analysis using anti-His antibody. (D) Binding of EhMIF to macrophages as determined by flow cytometry analysis. A total of 2 × 105 murine RAW-Blue macrophages were incubated at 4°C for 45 min with biotinylated EhMIF followed by streptavidin-Alexa Fluor 647 or with a streptavidin-Alexa Fluor 647-only control (SA-647). A red line represents the binding of biotinylated EhMIF to macrophages pretreated with anti-CD74 antibody (left) or binding of biotinylated EhMIF to macrophages in the presence of a 20-fold excess of mouse MIF (MMIF) (right). Data are representative of two independent experiments.
CD74 is expressed on the surfaces of a variety of mammalian cells, including macrophages. The binding of EhMIF to host macrophages was investigated by flow cytometry. Macrophages were incubated with biotinylated EhMIF, and binding of EhMIF to macrophages was observed (Fig. 2D). Macrophages pretreated with anti-CD74 antibodies resulted in a reduction of EhMIF binding. Anti-CD74 antibodies caused only a partial inhibition of EhMIF binding. This was not surprising, as MIF homologs have been shown to bind with other cell surface receptors, such as the chemokine receptors CXCR2 and CXCR4 (35). The receptor-binding similarities between EhMIF and mammalian MIF were further supported when incubation with a 20-fold excess of mouse MIF (MMIF) resulted in a pronounced reduction of EhMIF binding to macrophages (Fig. 2D).
EhMIF immunomodulates host macrophages.
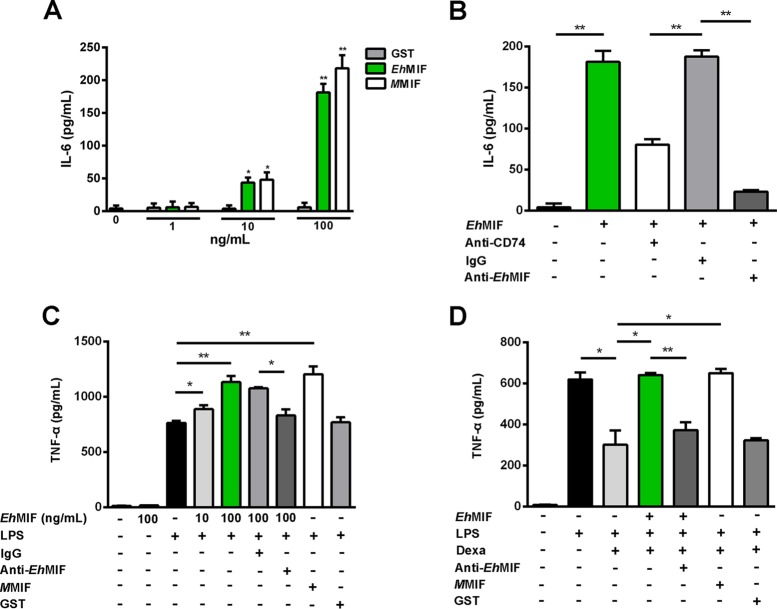
The binding of EhMIF to host macrophages suggested that EhMIF might be capable of interfering with or modulating macrophage activity. MIF homologs have been shown to promote inflammation by enhancing proinflammatory cytokine production (9, 11, 16, 17). For cytokine assays, we used endotoxin-free recombinant EhMIF (<1 pg LPS/μg protein). Cytokines (IL-6 and TNF-α) secreted into cell culture supernatants were collected after 8 h of stimulation with EhMIF. EhMIF induced IL-6 production in a dose-dependent manner (Fig. 3A). Treatment aimed at blocking CD74 or EhMIF (treatment with anti-EhMIF or anti-CD74 neutralizing antibodies) inhibited EhMIF-induced IL-6 production (Fig. 3B). Macrophages incubated with endotoxin-free EhMIF only did not show any significant increase in TNF-α secretion (Fig. 3C), in agreement with a previous study done with mammalian MIF (10).
FIG 3.
EhMIF immunomodulates macrophages. (A) EhMIF stimulates IL-6 production in a dose-dependent manner. RAW-Blue macrophages were stimulated with GST, EhMIF, or MMIF (0 to 100 ng/ml) for 8 h. (B) Inhibition of EhMIF-induced IL-6 production by neutralizing anti-CD74 and anti-EhMIF antibodies relative to that with the IgG control. Macrophages treated with anti-CD74 antibody or the IgG control were incubated with EhMIF (100 ng/ml) for 8 h. The amount of IL-6 secreted by macrophages into culture supernatants was measured after 8 h of stimulation with EhMIF preincubated with anti-EhMIF antibody or the IgG control. (C) EhMIF enhances TNF-α production of LPS-stimulated macrophages. Macrophages were incubated with EhMIF or EhMIF plus LPS (100 ng/ml). TNF-α secreted into culture supernatants was collected after 8 h and measured by ELISA. (D) EhMIF inhibits glucocorticoid-mediated suppression of TNF-α production. Macrophages were preincubated with dexamethasone (Dexa; 100 nM), with or without EhMIF (100 ng/ml), for 1 h and then stimulated with LPS (100 ng/ml). Culture supernatants were collected after 4 h, and TNF was quantified by ELISA. Data shown are means ± SD for triplicates from one experiment and are representative of three independent experiments. *, P < 0.005; **, P < 0.001.
There are two well-characterized proinflammatory effects of MIF. First, MIF enhances TNF-α production by LPS-stimulated immune cells. Second, MIF can override the anti-inflammatory activities of glucocorticoids (9–16). We tested the effect of EhMIF on TNF-α secretion by host macrophages with LPS stimulation. As shown in Fig. 3C, EhMIF was capable of augmenting TNF-α production by LPS-stimulated macrophages. To assess the effect of EhMIF on glucocorticoid-mediated immunosuppression, we pretreated macrophages with dexamethasone, with or without EhMIF, followed by stimulation with LPS. Dexamethasone inhibited TNF-α production by macrophages stimulated with LPS. Treatment of macrophages with EhMIF overcame dexamethasone inhibition of TNF secretion (Fig. 3D).
EhMIF demonstrated immunomodulatory activity comparable to that of MMIF. The immunomodulatory effects of EhMIF on macrophages were inhibited by a neutralizing anti-EhMIF antibody. We used recombinant GST, expressed, purified, and cleared of endotoxin under the same conditions as those for EhMIF, as a negative control. GST did not have any effect on cytokine secretion. Mouse and rabbit IgG controls had no effect on EhMIF activity (Fig. 3A to D).
Cytoplasmic localization of EhMIF.
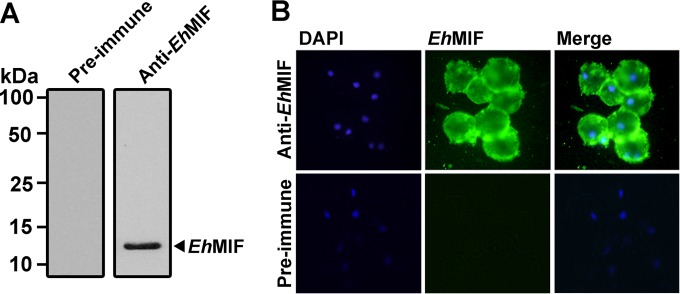
Host immune cells secrete MIF by a nonclassical pathway involving an ABC transporter. Once secreted, MIF then acts in an autocrine/paracrine fashion. Similar to mammalian MIF, EhMIF lacks a characteristic signal peptide (Fig. 1A), and putative ABC transporter genes can be found in the E. histolytica genome (29, 36). Cells constitutively express MIF protein, and high levels accumulate in the cytoplasm (37). We hypothesized that EhMIF was also localized to the parasite cytoplasm. Rabbit sera containing anti-EhMIF antibodies were generated. First, we determined if EhMIF was present in the soluble extract of trophozoites. Trophozoites were sonicated and centrifuged at 18,000 × g, and the supernatant (soluble cell extract) was then subjected to immunoblot analysis. Anti-EhMIF serum recognized a protein of 12 kDa, the predicted molecular mass of EhMIF, while no bands were seen with the preimmune serum (Fig. 4A). We next investigated the cellular localization of EhMIF. Trophozoites were fixed and permeabilized on polylysine-coated slides, and cellular localization of EhMIF was examined by immunofluorescence analysis using sera containing rabbit polyclonal anti-EhMIF antibodies. As shown in Fig. 4B, EhMIF was localized to the cytoplasm of trophozoites. Fluorescence was not seen in parasites treated with preimmune serum.
FIG 4.
Cytoplasmic localization of EhMIF. (A) Immunoblot analysis of soluble E. histolytica trophozoite extract, using serum containing anti-EhMIF antibodies or preimmune serum. Anti-EhMIF antibodies recognized a protein of 12 kDa, the predicted molecular mass of EhMIF. (B) Immunofluorescence detection of EhMIF in the cytoplasm of E. histolytica trophozoites. Trophozoites were permeabilized with 0.2% Triton X-100, and IFA was performed using serum containing rabbit anti-EhMIF antibodies or preimmune serum. Fluorescence staining was achieved with Alexa Fluor 488-conjugated secondary antibodies. Nuclei were stained with DAPI.
Anti-EhMIF antibodies are present in children's sera.
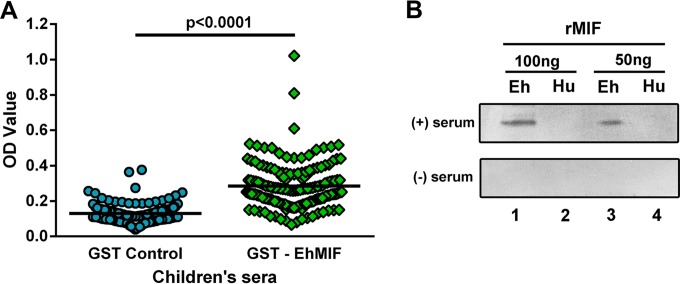
Previous studies have demonstrated the presence of antibodies against parasite MIF homologs, including those of the protozoan Plasmodium and the intestinal helminth Strongyloides, in the sera of individuals living in areas of endemicity (24, 32). We hypothesized that children infected with E. histolytica are capable of generating anti-EhMIF antibodies. To test this hypothesis, we first developed an ELISA to screen for anti-EhMIF antibodies in the sera of children living in an area where Entamoeba histolytica infection is endemic. We coated ELISA plates with a GST-EhMIF fusion protein, and GST-only plates were used as a control. Children's sera and anti-human IgG were added to the ELISA plates. The ELISA OD values were significantly higher in the presence of EhMIF (P < 0.0001), indicating the presence of anti-EhMIF IgG antibody in children's sera (Fig. 5A). Next, we used immunoblot analysis to confirm the presence of anti-EhMIF antibody in children's sera. As shown in Fig. 5B, children's sera positive for anti-EhMIF antibody reacted with recombinant EhMIF, and no reaction was seen with sera negative for anti-EhMIF antibody. We then evaluated whether anti-EhMIF antibody would cross-react with recombinant human MIF. Children's sera positive for anti-EhMIF antibody did not cross-react with human MIF (Fig. 5B).
FIG 5.
Anti-EhMIF antibodies are present in children's sera. (A) ELISA with sera from children exposed to E. histolytica. Children's sera were added to ELISA plates coated with GST-EhMIF or GST only. There was a statistically significant difference between the GST-EhMIF assay (mean = 0.28; SD = 0.13) and the GST control assay (mean = 0.13; SD = 0.05) (P < 0.0001; paired-sample t test). (B) Serum positive for anti-EhMIF antibody recognized EhMIF and did not cross-react with human MIF. Human (Hu) and E. histolytica (Eh) recombinant MIF (rMIF) proteins were analyzed at 100 ng and 50 ng by immunoblotting with anti-EhMIF antibody-positive and -negative sera.
DISCUSSION
The host immune response and the proinflammatory cytokine TNF-α, in particular, play a major role in the intestinal pathology induced by E. histolytica infection (5, 6, 38). Understanding the mechanisms by which E. histolytica drives gut inflammation is critical for the development of improved preventive and therapeutic strategies. In this report, we describe the first functional characterization of the E. histolytica homolog of the cytokine MIF. We produced recombinant EhMIF purified to remove endotoxin and showed that in a manner similar to that for mammalian MIF, EhMIF was capable of binding to host macrophages, at least in part through interaction with the MIF receptor, CD74. We went on to show that EhMIF induced IL-6 production, amplified the secretion of TNF-α by LPS-stimulated macrophages, and reversed the glucocorticoid inhibition of TNF-α secretion.
Previously, coculture of human cells with E. histolytica trophozoites was shown to increase expression and secretion of proinflammatory cytokines, including IL-6 (39). Our finding that EhMIF induced IL-6 secretion is consistent with previous studies of MIF homologs (16, 17, 25, 40–42). IL-6 is a proinflammatory cytokine, and its main sources in the intestine are macrophages. IL-6 plays a crucial role in the uncontrolled inflammation of the intestinal mucosa that is characteristic of IBD (43, 44). These findings support the concept that EhMIF might contribute to the immunopathology of intestinal amebiasis.
Blocking of CD74 decreased EhMIF-induced IL-6 secretion. Naturally resistant B6 mice become highly susceptible to amebic colitis after disruption of the anti-inflammatory cytokine IL-10 gene. It is interesting that CD74 is upregulated in these IL-10-deficient C57/B6 mice and also in inflammatory bowel disease (45, 46). One could postulate that host CD74 overexpression would enhance EhMIF activity, which would partly explain the increased susceptibility of IL-10−/− mice.
LPS is a major component of the Gram-negative bacterial outer membrane. It is possible that during amebic colitis, LPS may enter the host lamina propria through at least two paths. First, E. histolytica infection results in disruption of the mucosal barrier, which might lead to infiltration of bacteria into the lamina propria. Second, trophozoites feed on gut flora; hence, invading parasites could carry bacterial products, including LPS (46, 47). As reported previously, EhMIF in combination with LPS increased TNF-α production by macrophages in vitro. The concentration of TNF-α produced plays a pivotal role in gut inflammation, and an excess of TNF-α can result in damage to intestinal tissue (48). Roger et al. demonstrated that MIF enhances the host response to LPS by upregulating Toll-like receptor 4 (TLR4), the receptor for LPS (9).
The gut mucosa contains the largest number of immune cells in the body, and tight regulation of the mucosal immune response is required to maintain intestinal homeostasis. Glucocorticoids are locally synthesized in the intestine and play an important role in downregulating the inflammatory immune response and mediating repair of a damaged mucosa (49–55). Mitogen-activated protein kinase (MAPK) phosphatase 1 (MKP-1) is induced by glucocorticoids to dephosphorylate and therefore deactivate MAPK activity in response to proinflammatory stimuli (e.g., LPS). MIF inhibits glucocorticoid-induced MKP-1 expression and inhibition of TNF-α production by LPS-stimulated immune cells (15, 54, 56). It is reasonable to deduce that the glucocorticoid-overriding activity of EhMIF disrupts the tightly regulated immune response, creating a proinflammatory state in the gut during E. histolytica infection.
It appears counterintuitive that parasite genomes would encode a proinflammatory cytokine, but EhMIF may play several important roles during the parasite life cycle. It has been shown that TNF-α has a chemotactic effect on E. histolytica; hence, it is possible that by enhancing TNF-α, EhMIF promotes parasite invasion (57, 58). Also, TNF-α promotes adherence of E. histolytica to host cells (59). It was recently shown that the Plasmodium MIF cytokine interferes with the development of immunological memory by inducing the development of short-lived effector cells rather than memory cells (16). EhMIF might therefore perform a function similar to that of Plasmodium MIF, rendering the host susceptible to reinfection by the parasite. Furthermore, E. histolytica has developed an impressive number of mechanisms to evade the host immune response (4). For example, the amebic surface protein peroxiredoxin possesses antioxidant activity, which protects the parasite from immune cell reactive oxygen species (60). In addition to cytokine functions, mammalian MIF, through interaction with the JAB-1 protein, coordinates cell cycle checkpoints promoting DNA repair (61, 62). Since a putative JAB-1 gene homolog can be found in the Entamoeba genome (29), EhMIF might have a physiological function involving cell cycle regulation in amebae. It would be interesting in future studies to determine whether an EhMIF–JAB-1 functional interaction exists in replicating trophozoites.
The present study demonstrated that an anti-EhMIF IgG antibody present in the sera of children recognized EhMIF but did not cross-react with human MIF. Although EhMIF and human MIF share similar structures and biological functions, their relatively low amino acid sequence identity (28%) allows for different immunoreactive epitopes. This finding supports the possibility of developing immunotherapy against EhMIF without affecting normal human MIF activity. Immunized rats possessing antibodies against the MIF homolog of the intestinal parasite Strongyloides were previously shown to have a reduced worm burden compared to that in nonimmunized rats, suggesting a potential protective role of antibodies against parasitic MIF homologs (32). However, it is not known whether anti-EhMIF antibodies protect against amebiasis.
In conclusion, our study suggests that the interactions between the E. histolytica homolog of the cytokine MIF and host immune cells leads to an exaggerated inflammatory response and may contribute to disease. This study also generates the hypothesis that neutralization of EhMIF may serve as potential immunotherapy against this devastating disease. We plan to examine whether antibodies against EhMIF can prevent and/or attenuate disease in an amebic mouse model and whether anti-EhMIF antibodies present in children's sera are associated with protection.
ACKNOWLEDGMENTS
We thank the families in Mirpur, Dhaka, Bangladesh, for their participation in these studies.
This work was supported by NIH grant R01 AI043596 (W.A.P.) and Biodefense Research Training and Career Development grant 5T32AI055432-10 (S.N.M.).
Footnotes
Published ahead of print 12 May 2014
REFERENCES
- 1.Black RE, Cousens S, Johnson HL, Lawn JE, Rudan I, Bassani DG, Jha P, Campbell H, Walker CF, Cibulskis R, Eisele T, Liu L, Mathers C. 2010. Global, regional, and national causes of child mortality in 2008: a systematic analysis. Lancet 375:1969–1987. 10.1016/S0140-6736(10)60549-1 [DOI] [PubMed] [Google Scholar]
- 2.Kotloff KL, Nataro JP, Blackwelder WC, Nasrin D, Farag TH, Panchalingam S, Wu Y, Sow SO, Sur D, Breiman RF, Faruque AS, Zaidi AK, Saha D, Alonso PL, Tamboura B, Sanogo D, Onwuchekwa U, Manna B, Ramamurthy T, Kanungo S, Ochieng JB, Omore R, Oundo JO, Hossain A, Das SK, Ahmed S, Qureshi S, Quadri F, Adegbola RA, Antonio M, Hossain MJ, Akinsola A, Mandomando I, Nhampossa T, Acacio S, Biswas K, O'Reilly CE, Mintz ED, Berkeley LY, Muhsen K, Sommerfelt H, Robins-Browne RM, Levine MM. 2013. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): a prospective, case-control study. Lancet 382:209–222. 10.1016/S0140-6736(13)60844-2 [DOI] [PubMed] [Google Scholar]
- 3.Taniuchi M, Sobuz SU, Begum S, Platts-Mills JA, Liu J, Yang Z, Wang XQ, Petri WA, Jr, Haque R, Houpt ER. 2013. Etiology of diarrhea in Bangladeshi infants in the first year of life analyzed using molecular methods. J. Infect. Dis. 208:1794–1802. 10.1093/infdis/jit507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moonah SN, Jiang NM, Petri WA., Jr 2013. Host immune response to intestinal amebiasis. PLoS Pathog. 9:e1003489. 10.1371/journal.ppat.1003489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peterson KM, Shu J, Duggal P, Haque R, Mondal D, Petri WA., Jr 2010. Association between TNF-alpha and Entamoeba histolytica diarrhea. Am. J. Trop. Med. Hyg. 82:620–625. 10.4269/ajtmh.2010.09-0493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang Z, Mahajan S, Zhang X, Stanley SL., Jr 2003. Tumor necrosis factor alpha is a key mediator of gut inflammation seen in amebic colitis in human intestine in the SCID mouse-human intestinal xenograft model of disease. Infect. Immun. 71:5355–5359. 10.1128/IAI.71.9.5355-5359.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Helk E, Bernin H, Ernst T, Ittrich H, Jacobs T, Heeren J, Tacke F, Tannich E, Lotter H. 2013. TNFalpha-mediated liver destruction by Kupffer cells and Ly6Chi monocytes during Entamoeba histolytica infection. PLoS Pathog. 9:e1003096. 10.1371/journal.ppat.1003096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Calandra T, Roger T. 2003. Macrophage migration inhibitory factor: a regulator of innate immunity. Nat. Rev. Immunol. 3:791–800. 10.1038/nri1200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roger T, David J, Glauser MP, Calandra T. 2001. MIF regulates innate immune responses through modulation of Toll-like receptor 4. Nature 414:920–924. 10.1038/414920a [DOI] [PubMed] [Google Scholar]
- 10.Kudrin A, Scott M, Martin S, Chung CW, Donn R, McMaster A, Ellison S, Ray D, Ray K, Binks M. 2006. Human macrophage migration inhibitory factor: a proven immunomodulatory cytokine? J. Biol. Chem. 281:29641–29651. 10.1074/jbc.M601103200 [DOI] [PubMed] [Google Scholar]
- 11.Alam A, Haldar S, Thulasiram HV, Kumar R, Goyal M, Iqbal MS, Pal C, Dey S, Bindu S, Sarkar S, Pal U, Maiti NC, Bandyopadhyay U. 2012. Novel anti-inflammatory activity of epoxyazadiradione against macrophage migration inhibitory factor: inhibition of tautomerase and proinflammatory activities of macrophage migration inhibitory factor. J. Biol. Chem. 287:24844–24861. 10.1074/jbc.M112.341321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Calandra T, Bernhagen J, Metz CN, Spiegel LA, Bacher M, Donnelly T, Cerami A, Bucala R. 1995. MIF as a glucocorticoid-induced modulator of cytokine production. Nature 377:68–71. 10.1038/377068a0 [DOI] [PubMed] [Google Scholar]
- 13.Merk M, Zierow S, Leng L, Das R, Du X, Schulte W, Fan J, Lue H, Chen Y, Xiong H, Chagnon F, Bernhagen J, Lolis E, Mor G, Lesur O, Bucala R. 2011. The D-dopachrome tautomerase (DDT) gene product is a cytokine and functional homolog of macrophage migration inhibitory factor (MIF). Proc. Natl. Acad. Sci. U. S. A. 108:E577–E585. 10.1073/pnas.1102941108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Senter PD, Al-Abed Y, Metz CN, Benigni F, Mitchell RA, Chesney J, Han J, Gartner CG, Nelson SD, Todaro GJ, Bucala R. 2002. Inhibition of macrophage migration inhibitory factor (MIF) tautomerase and biological activities by acetaminophen metabolites. Proc. Natl. Acad. Sci. U. S. A. 99:144–149. 10.1073/pnas.011569399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roger T, Chanson AL, Knaup-Reymond M, Calandra T. 2005. Macrophage migration inhibitory factor promotes innate immune responses by suppressing glucocorticoid-induced expression of mitogen-activated protein kinase phosphatase-1. Eur. J. Immunol. 35:3405–3413. 10.1002/eji.200535413 [DOI] [PubMed] [Google Scholar]
- 16.Sun T, Holowka T, Song Y, Zierow S, Leng L, Chen Y, Xiong H, Griffith J, Nouraie M, Thuma PE, Lolis E, Janse CJ, Gordeuk VR, Augustijn K, Bucala R. 2012. A Plasmodium-encoded cytokine suppresses T-cell immunity during malaria. Proc. Natl. Acad. Sci. U. S. A. 109:E2117–E2126. 10.1073/pnas.1206573109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Jong YP, Abadia-Molina AC, Satoskar AR, Clarke K, Rietdijk ST, Faubion WA, Mizoguchi E, Metz CN, Alsahli M, ten Hove T, Keates AC, Lubetsky JB, Farrell RJ, Michetti P, van Deventer SJ, Lolis E, David JR, Bhan AK, Terhorst C. 2001. Development of chronic colitis is dependent on the cytokine MIF. Nat. Immunol. 2:1061–1066. 10.1038/ni720 [DOI] [PubMed] [Google Scholar]
- 18.Leng L, Metz CN, Fang Y, Xu J, Donnelly S, Baugh J, Delohery T, Chen Y, Mitchell RA, Bucala R. 2003. MIF signal transduction initiated by binding to CD74. J. Exp. Med. 197:1467–1476. 10.1084/jem.20030286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oliver J, Marquez A, Gomez-Garcia M, Martinez A, Mendoza JL, Vilchez JR, Lopez-Nevot MA, Pinero A, de la Concha EG, Nieto A, Urcelay E, Martin J. 2007. Association of the macrophage migration inhibitory factor gene polymorphisms with inflammatory bowel disease. Gut 56:150–151. 10.1136/gut.2006.107649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang H, Ma L, Dong LQ, Shu C, Xu JL. 2013. Association of the macrophage migration inhibitory factor gene −173G/C polymorphism with inflammatory bowel disease: a meta-analysis of 4296 subjects. Gene 526:228–231. 10.1016/j.gene.2013.05.012 [DOI] [PubMed] [Google Scholar]
- 21.Nishihira J, Mitsuyama K. 2009. Overview of the role of macrophage migration inhibitory factor (MIF) in inflammatory bowel disease. Curr. Pharm. Des. 15:2104–2109. 10.2174/138161209788489113 [DOI] [PubMed] [Google Scholar]
- 22.Ohkawara T, Koyama Y, Onodera S, Takeda H, Kato M, Asaka M, Nishihira J. 2011. DNA vaccination targeting macrophage migration inhibitory factor prevents murine experimental colitis. Clin. Exp. Immunol. 163:113–122. 10.1111/j.1365-2249.2010.04277.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Augustijn KD, Kleemann R, Thompson J, Kooistra T, Crawford CE, Reece SE, Pain A, Siebum AH, Janse CJ, Waters AP. 2007. Functional characterization of the Plasmodium falciparum and P. berghei homologues of macrophage migration inhibitory factor. Infect. Immun. 75:1116–1128. 10.1128/IAI.00902-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cordery DV, Kishore U, Kyes S, Shafi MJ, Watkins KR, Williams TN, Marsh K, Urban BC. 2007. Characterization of a Plasmodium falciparum macrophage-migration inhibitory factor homologue. J. Infect. Dis. 195:905–912. 10.1086/511309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shao D, Zhong X, Zhou YF, Han Z, Lin Y, Wang Z, Bu L, Zhang L, Su XD, Wang H. 2010. Structural and functional comparison of MIF ortholog from Plasmodium yoelii with MIF from its rodent host. Mol. Immunol. 47:726–737. 10.1016/j.molimm.2009.10.037 [DOI] [PubMed] [Google Scholar]
- 26.Miller JL, Harupa A, Kappe SH, Mikolajczak SA. 2012. Plasmodium yoelii macrophage migration inhibitory factor is necessary for efficient liver-stage development. Infect. Immun. 80:1399–1407. 10.1128/IAI.05861-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kamir D, Zierow S, Leng L, Cho Y, Diaz Y, Griffith J, McDonald C, Merk M, Mitchell RA, Trent J, Chen Y, Kwong YK, Xiong H, Vermeire J, Cappello M, McMahon-Pratt D, Walker J, Bernhagen J, Lolis E, Bucala R. 2008. A Leishmania ortholog of macrophage migration inhibitory factor modulates host macrophage responses. J. Immunol. 180:8250–8261. 10.4049/jimmunol.180.12.8250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sommerville C, Richardson JM, Williams RA, Mottram JC, Roberts CW, Alexander J, Henriquez FL. 2013. Biochemical and immunological characterization of Toxoplasma gondii macrophage migration inhibitory factor. J. Biol. Chem. 288:12733–12741. 10.1074/jbc.M112.419911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Loftus B, Anderson I, Davies R, Alsmark UC, Samuelson J, Amedeo P, Roncaglia P, Berriman M, Hirt RP, Mann BJ, Nozaki T, Suh B, Pop M, Duchene M, Ackers J, Tannich E, Leippe M, Hofer M, Bruchhaus I, Willhoeft U, Bhattacharya A, Chillingworth T, Churcher C, Hance Z, Harris B, Harris D, Jagels K, Moule S, Mungall K, Ormond D, Squares R, Whitehead S, Quail MA, Rabbinowitsch E, Norbertczak H, Price C, Wang Z, Guillen N, Gilchrist C, Stroup SE, Bhattacharya S, Lohia A, Foster PG, Sicheritz-Ponten T, Weber C, Singh U, Mukherjee C, El-Sayed NM, Petri WA, Jr, Clark CG, Embley TM, Barrell B, Fraser CM, Hall N. 2005. The genome of the protist parasite Entamoeba histolytica. Nature 433:865–868. 10.1038/nature03291 [DOI] [PubMed] [Google Scholar]
- 30.Ehrenkaufer GM, Haque R, Hackney JA, Eichinger DJ, Singh U. 2007. Identification of developmentally regulated genes in Entamoeba histolytica: insights into mechanisms of stage conversion in a protozoan parasite. Cell. Microbiol. 9:1426–1444. 10.1111/j.1462-5822.2006.00882.x [DOI] [PubMed] [Google Scholar]
- 31.Dobson SE, Augustijn KD, Brannigan JA, Schnick C, Janse CJ, Dodson EJ, Waters AP, Wilkinson AJ. 2009. The crystal structures of macrophage migration inhibitory factor from Plasmodium falciparum and Plasmodium berghei. Protein Sci. 18:2578–2591. 10.1002/pro.263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Younis AE, Soblik H, Ajonina-Ekoti I, Erttmann KD, Luersen K, Liebau E, Brattig NW. 2012. Characterization of a secreted macrophage migration inhibitory factor homologue of the parasitic nematode Strongyloides acting at the parasite-host cell interface. Microbes Infect. 14:279–289. 10.1016/j.micinf.2011.09.006 [DOI] [PubMed] [Google Scholar]
- 33.Haque R, Ali IM, Sack RB, Farr BM, Ramakrishnan G, Petri WA., Jr 2001. Amebiasis and mucosal IgA antibody against the Entamoeba histolytica adherence lectin in Bangladeshi children. J. Infect. Dis. 183:1787–1793. 10.1086/320740 [DOI] [PubMed] [Google Scholar]
- 34.Singh G. 2007. Determination of cutoff score for a diagnostic test. Internet J. Lab. Med. 2:1 http://ispub.com/IJLM/2/1/9884 [Google Scholar]
- 35.Bernhagen J, Krohn R, Lue H, Gregory JL, Zernecke A, Koenen RR, Dewor M, Georgiev I, Schober A, Leng L, Kooistra T, Fingerle-Rowson G, Ghezzi P, Kleemann R, McColl SR, Bucala R, Hickey MJ, Weber C. 2007. MIF is a noncognate ligand of CXC chemokine receptors in inflammatory and atherogenic cell recruitment. Nat. Med. 13:587–596. 10.1038/nm1567 [DOI] [PubMed] [Google Scholar]
- 36.Flieger O, Engling A, Bucala R, Lue H, Nickel W, Bernhagen J. 2003. Regulated secretion of macrophage migration inhibitory factor is mediated by a non-classical pathway involving an ABC transporter. FEBS Lett. 551:78–86. 10.1016/S0014-5793(03)00900-1 [DOI] [PubMed] [Google Scholar]
- 37.Greven D, Leng L, Bucala R. 2010. Autoimmune diseases: MIF as a therapeutic target. Expert Opin. Ther. Targets 14:253–264. 10.1517/14728220903551304 [DOI] [PubMed] [Google Scholar]
- 38.Kissoon-Singh V, Moreau F, Trusevych E, Chadee K. 2013. Entamoeba histolytica exacerbates epithelial tight junction permeability and proinflammatory responses in Muc2(−/−) mice. Am. J. Pathol. 182:852–865. 10.1016/j.ajpath.2012.11.035 [DOI] [PubMed] [Google Scholar]
- 39.Eckmann L, Reed SL, Smith JR, Kagnoff MF. 1995. Entamoeba histolytica trophozoites induce an inflammatory cytokine response by cultured human cells through the paracrine action of cytolytically released interleukin-1 alpha. J. Clin. Invest. 96:1269–1279. 10.1172/JCI118161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jang SI, Lillehoj HS, Lee SH, Kim DK, Pages M, Hong YH, Min W, Lillehoj EP. 2011. Distinct immunoregulatory properties of macrophage migration inhibitory factors encoded by Eimeria parasites and their chicken host. Vaccine 29:8998–9004. 10.1016/j.vaccine.2011.09.038 [DOI] [PubMed] [Google Scholar]
- 41.Bai F, Asojo OA, Cirillo P, Ciustea M, Ledizet M, Aristoff PA, Leng L, Koski RA, Powell TJ, Bucala R, Anthony KG. 2012. A novel allosteric inhibitor of macrophage migration inhibitory factor (MIF). J. Biol. Chem. 287:30653–30663. 10.1074/jbc.M112.385583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Miska K, Kim S, Fetterer R, Dalloul R, Jenkins M. 2013. Macrophage migration inhibitory factor (MIF) of the protozoan parasite Eimeria influences the components of the immune system of its host, the chicken. Parasitol. Res. 112:1935–1944. 10.1007/s00436-013-3345-z [DOI] [PubMed] [Google Scholar]
- 43.Atreya R, Neurath M. 2005. Involvement of IL-6 in the pathogenesis of inflammatory bowel disease and colon cancer. Clin. Rev. Allerg. Immunol. 28:187–195. 10.1385/CRIAI:28:3:187 [DOI] [PubMed] [Google Scholar]
- 44.Bernardo D, Vallejo-Díez S, Mann ER, Al-Hassi HO, Martínez-Abad B, Montalvillo E, Tee CT, Murugananthan AU, Núñez H, Peake STC, Hart AL, Fernández-Salazar L, Garrote JA, Arranz E, Knight SC. 2012. IL-6 promotes immune responses in human ulcerative colitis and induces a skin-homing phenotype in the dendritic cells and T cells they stimulate. Eur. J. Immunol. 42:1337–1353. 10.1002/eji.201142327 [DOI] [PubMed] [Google Scholar]
- 45.Hamano S, Asgharpour A, Stroup SE, Wynn TA, Leiter EH, Houpt E. 2006. Resistance of C57BL/6 mice to amoebiasis is mediated by nonhemopoietic cells but requires hemopoietic IL-10 production. J. Immunol. 177:1208–1213. 10.4049/jimmunol.177.2.1208 [DOI] [PubMed] [Google Scholar]
- 46.Lawrance IC, Fiocchi C, Chakravarti S. 2001. Ulcerative colitis and Crohn's disease: distinctive gene expression profiles and novel susceptibility candidate genes. Hum. Mol. Genet. 10:445–456. 10.1093/hmg/10.5.445 [DOI] [PubMed] [Google Scholar]
- 47.Mukherjee C, Clark CG, Lohia A. 2008. Entamoeba shows reversible variation in ploidy under different growth conditions and between life cycle phases. PLoS Negl. Trop. Dis. 2:e281. 10.1371/journal.pntd.0000281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nielsen OH, Ainsworth MA. 2013. Tumor necrosis factor inhibitors for inflammatory bowel disease. N. Engl. J. Med. 369:754–762. 10.1056/NEJMct1209614 [DOI] [PubMed] [Google Scholar]
- 49.Noti M, Corazza N, Tuffin G, Schoonjans K, Brunner T. 2010. Lipopolysaccharide induces intestinal glucocorticoid synthesis in a TNFalpha-dependent manner. FASEB J. 24:1340–1346. 10.1096/fj.09-140913 [DOI] [PubMed] [Google Scholar]
- 50.Atanasov AG, Leiser D, Roesselet C, Noti M, Corazza N, Schoonjans K, Brunner T. 2008. Cell cycle-dependent regulation of extra-adrenal glucocorticoid synthesis in murine intestinal epithelial cells. FASEB J. 22:4117–4125. 10.1096/fj.08-114157 [DOI] [PubMed] [Google Scholar]
- 51.Mueller M, Atanasov A, Cima I, Corazza N, Schoonjans K, Brunner T. 2007. Differential regulation of glucocorticoid synthesis in murine intestinal epithelial versus adrenocortical cell lines. Endocrinology 148:1445–1453. 10.1210/en.2006-0591 [DOI] [PubMed] [Google Scholar]
- 52.Coste A, Dubuquoy L, Barnouin R, Annicotte JS, Magnier B, Notti M, Corazza N, Antal MC, Metzger D, Desreumaux P, Brunner T, Auwerx J, Schoonjans K. 2007. LRH-1-mediated glucocorticoid synthesis in enterocytes protects against inflammatory bowel disease. Proc. Natl. Acad. Sci. U. S. A. 104:13098–13103. 10.1073/pnas.0702440104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mueller M, Cima I, Noti M, Fuhrer A, Jakob S, Dubuquoy L, Schoonjans K, Brunner T. 2006. The nuclear receptor LRH-1 critically regulates extra-adrenal glucocorticoid synthesis in the intestine. J. Exp. Med. 203:2057–2062. 10.1084/jem.20060357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Aeberli D, Yang Y, Mansell A, Santos L, Leech M, Morand EF. 2006. Endogenous macrophage migration inhibitory factor modulates glucocorticoid sensitivity in macrophages via effects on MAP kinase phosphatase-1 and p38 MAP kinase. FEBS Lett. 580:974–981. 10.1016/j.febslet.2006.01.027 [DOI] [PubMed] [Google Scholar]
- 55.Cima I, Corazza N, Dick B, Fuhrer A, Herren S, Jakob S, Ayuni E, Mueller C, Brunner T. 2004. Intestinal epithelial cells synthesize glucocorticoids and regulate T cell activation. J. Exp. Med. 200:1635–1646. 10.1084/jem.20031958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liu Y, Shepherd EG, Nelin LD. 2007. MAPK phosphatases—regulating the immune response. Nat. Rev. Immunol. 7:202–212. 10.1038/nri2035 [DOI] [PubMed] [Google Scholar]
- 57.Blazquez S, Zimmer C, Guigon G, Olivo-Marin JC, Guillen N, Labruyere E. 2006. Human tumor necrosis factor is a chemoattractant for the parasite Entamoeba histolytica. Infect. Immun. 74:1407–1411. 10.1128/IAI.74.2.1407-1411.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Blazquez S, Guigon G, Weber C, Syan S, Sismeiro O, Coppee JY, Labruyere E, Guillen N. 2008. Chemotaxis of Entamoeba histolytica towards the pro-inflammatory cytokine TNF is based on PI3K signalling, cytoskeleton reorganization and the galactose/N-acetylgalactosamine lectin activity. Cell. Microbiol. 10:1676–1686. 10.1111/j.1462-5822.2008.01158.x [DOI] [PubMed] [Google Scholar]
- 59.Pillai DR, Kain KC. 2005. Entamoeba histolytica: identification of a distinct beta2 integrin-like molecule with a potential role in cellular adherence. Exp. Parasitol. 109:135–142. 10.1016/j.exppara.2004.12.007 [DOI] [PubMed] [Google Scholar]
- 60.Davis PH, Zhang X, Guo J, Townsend RR, Stanley SL., Jr 2006. Comparative proteomic analysis of two Entamoeba histolytica strains with different virulence phenotypes identifies peroxiredoxin as an important component of amoebic virulence. Mol. Microbiol. 61:1523–1532. 10.1111/j.1365-2958.2006.05344.x [DOI] [PubMed] [Google Scholar]
- 61.Kleemann R, Hausser A, Geiger G, Mischke R, Burger-Kentischer A, Flieger O, Johannes FJ, Roger T, Calandra T, Kapurniotu A, Grell M, Finkelmeier D, Brunner H, Bernhagen J. 2000. Intracellular action of the cytokine MIF to modulate AP-1 activity and the cell cycle through Jab1. Nature 408:211–216. 10.1038/35041591 [DOI] [PubMed] [Google Scholar]
- 62.Burger-Kentischer A, Finkelmeier D, Thiele M, Schmucker J, Geiger G, Tovar GE, Bernhagen J. 2005. Binding of JAB1/CSN5 to MIF is mediated by the MPN domain but is independent of the JAMM motif. FEBS Lett. 579:1693–1701. 10.1016/j.febslet.2005.01.080 [DOI] [PubMed] [Google Scholar]