FIG 1.
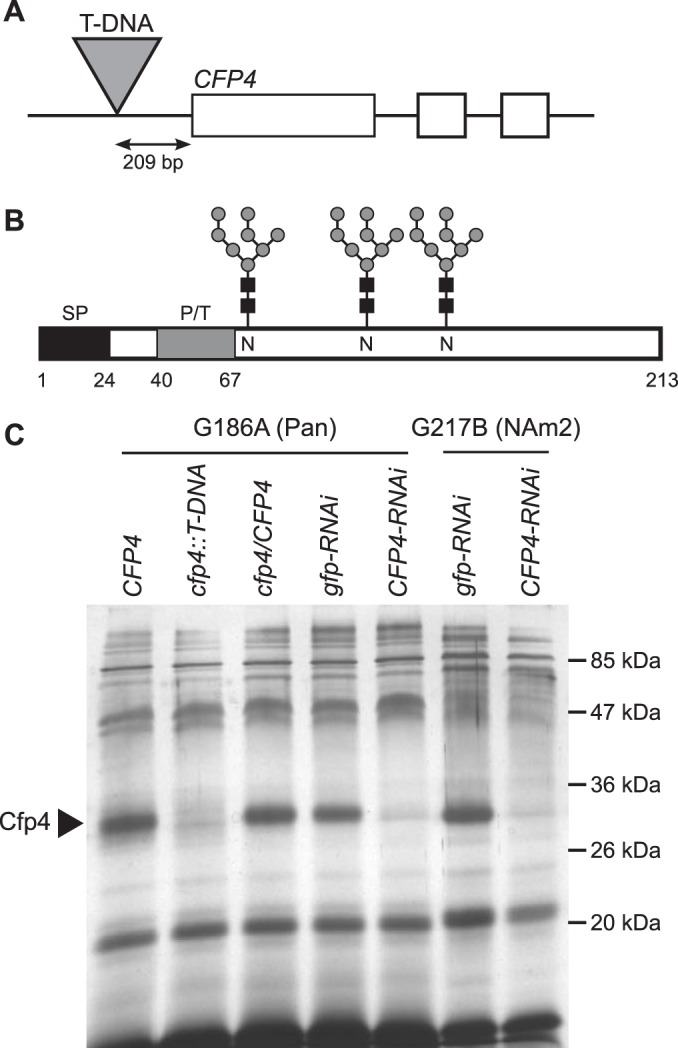
Generation of Cfp4-depleted Histoplasma strains. (A) Scaled depiction of the CFP4 gene, which consists of 3 exons (boxes), and the cfp4::T-DNA mutant (strain OSU6) in which a T-DNA insertion is located 209 bp upstream of the initiation codon. (B) Representation of the primary structure of the Cfp4 protein. Schematic indicates the secretion signal peptide (SP; black box), the proline/threonine-rich region (P/T; gray box), and three sites of N-linked glycosylation (glycan symbols) and the respective asparagines (N). Numbers indicate the amino acid residues. (C) Analysis of culture filtrates showing depletion of Cfp4 protein by T-DNA insertion and by RNAi. Culture filtrates were collected from G186A (Panama) and G217B (NAm2) background strains. Culture filtrate proteins were first treated with PNGase F to remove N-linked glycans, separated by one-dimensional SDS-PAGE, and visualized by silver staining. Strains analyzed include the Cfp4-producing parent strain (CFP4; strain WU8), the strain with a T-DNA insertion in the CFP4 gene promoter (cfp4::T-DNA; strain OSU6), and the complemented strain (cfp4/CFP4; strain OSU44). Cfp4 was also depleted by RNAi in both backgrounds, as shown by strains harboring plasmids for knockdown of the unrelated gfp gene (gfp-RNAi; strains OSU100 and OSU75) or CFP4 (CFP4-RNAi; strains OSU63 and OSU87).
