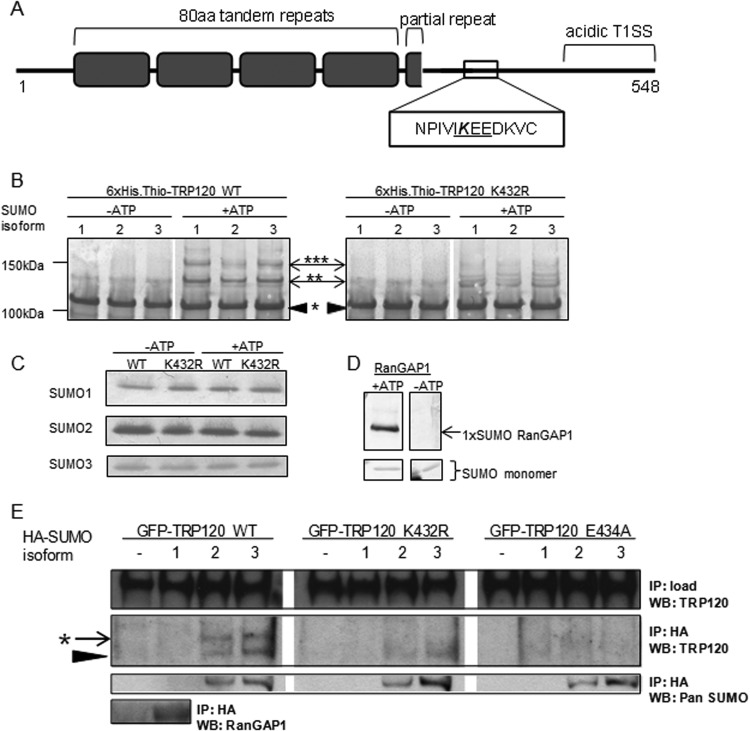
FIG 3.
E. chaffeensis TRP120 encompasses a carboxy-terminal canonical consensus SUMO motif that is modified in vitro and in human cells. (A) Illustration of TRP120 protein domains, including tandem repeats (gray boxes) and an acidic T1SS at the carboxy terminus, highlighting the canonical consensus SUMO motif (ψKxD/E, where ψ is a hydrophobic residue and “x” is any residue), in which lysine 432 is predicted to be SUMO conjugated. (B) In vitro SUMOylation assay (Enzo Life Sciences) of recombinant 6×His-thioredoxin-tagged wild-type TRP120 or the K432R mutant, with or without ATP. Western blot analysis performed with anti-TRP120 shows higher-molecular-weight laddering of wild-type TRP120 in the presence of ATP, indicative of multiple SUMO modification events. *, unmodified TRP120; **, mono-SUMOylation; ***, poly- or multi-SUMOylation. Representative data are shown (n = 3). TRP120 K432R yielded a diminished laddering pattern, demonstrating that the lysine residue is the primary site of SUMO conjugation in vitro. (C) Immunoblot performed with SUMO antibodies, demonstrating equal loading of each recombinant SUMO isoform for conditions with and without ATP. (D) Purified recombinant RanGAP1 was used as a positive control for in vitro SUMOylation. Immunoblot analysis with anti-pan-SUMO demonstrates a single SUMOylation of RanGAP1, as previously reported (87). (E) In cellular studies, GFP-TRP120 WT or the K432R or E434A mutant was ectopically expressed alone or with HA-SUMO isoforms in HeLa cells. Anti-HA immunoprecipitation (IP) and analysis by anti-TRP120 immunoblotting (WB) demonstrated that wild-type TRP120 was conjugated by a single SUMO2/3 protein in cells (*), while TRP120 K432R and TRP120 E434A were not modified. Representative data are shown (n = 6). Unmodified TRP120 (black arrowhead) coimmunoprecipitated with HA-SUMO2 and HA-SUMO3, suggesting a noncovalent interaction with SUMO or SUMO-modified proteins.