FIG 4.
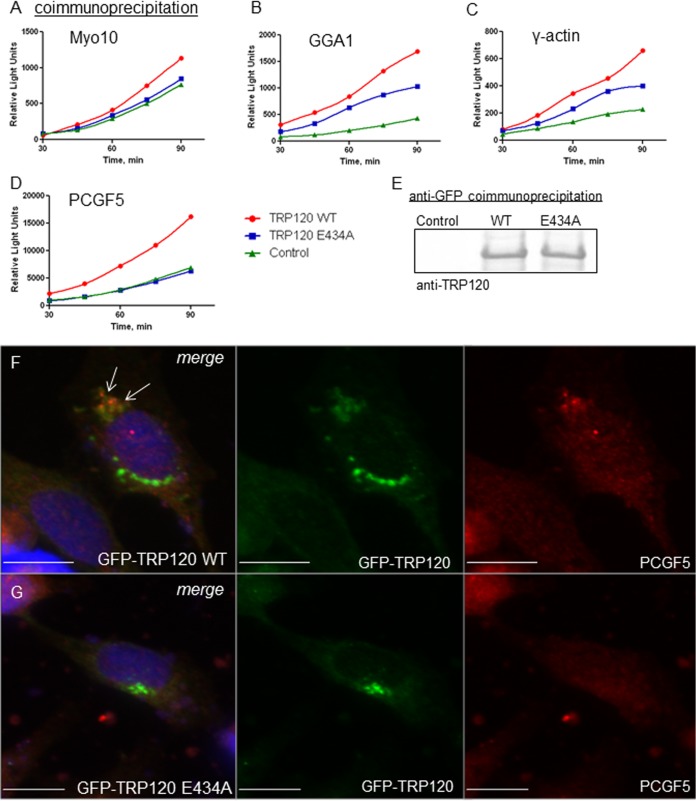
Disruption of TRP120 SUMOylation perturbs interactions with host proteins. HeLa cells were transiently cotransfected with GFP-vector (control), GFP-TRP120 wild type (WT), or GFP-TRP120 E434A and ProLabel-tagged host protein domains (as previously reported [7]). Coimmunoprecipitation was performed with anti-GFP antibody at 24 h posttransfection, and ProLabel activity was measured (relative light units) over 90 min to determine the relative impact of SUMOylation on TRP120-host interactions. Representative data are shown (n = 3); differences in absolute relative light units varied between experiments. (A) γ-Actin; (B) GGA1; (C) Myo10; (D) PCGF5. (E) Anti-TRP120 immunoblot analysis of GFP coimmunoprecipitations demonstrates that wild-type TRP120 and the SUMO-null mutant (E434A) were pulled down with similar efficiencies and that pulldown efficiency did not contribute to observed differences in ProLabel activity. Colocalization of ectopically expressed GFP-TRP120 WT (green; F) and native PCGF5 (red) was observed in HeLa cells by use of immunofluorescence microscopy (white arrows), while colocalization was not observed with the TRP120 E434A SUMO-null mutant (green; G). Bars, 10 μm.