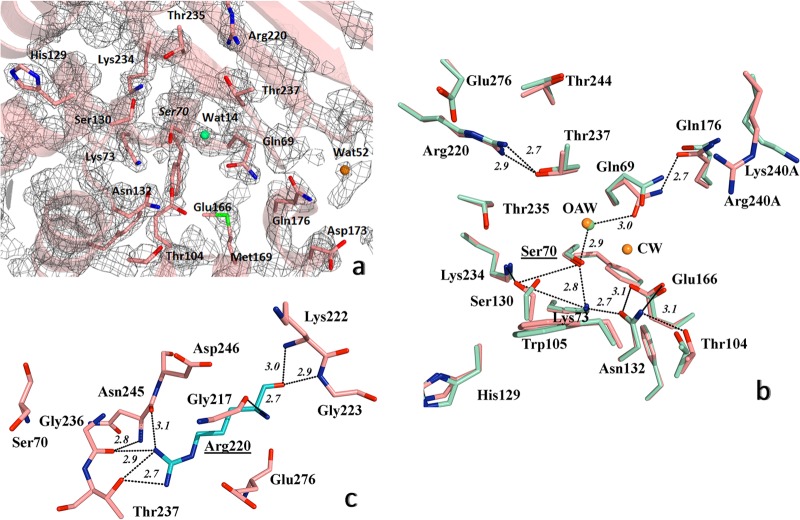
FIG 3.
Detailed view of the structure of active site of PER-2 β-lactamase. (a) 2FoFc map contoured at 1.5σ is shown in gray around the most important amino acid residues within the active site; oxyanion water molecule is shown as a green sphere, and additional water molecules in orange (see the Results and Discussion for details). (b) Comparative active site organization of PER-2 (pink) and PER-1 (cyan), indicating the main hydrogen bonds (black dashed lines) implicated in the stabilization of the active site of PER-2, including the oxyanion water molecules (OAW) (green for PER-2 and orange for PER-1) and the catalytic water of PER-1 (CW) (orange), and the network Ser70-Gln69-Wat14-Thr237-Arg220 (see Results and Discussion for details); for visual convenience, only the hydrogen bonds for PER-2 were shown. (c) Position and occupancy of Arg220 in PER-2, allowing the creation of a unique network of hydrogen bonds with neighboring residues like Gly236, Thr237, Asn245, and Glu276, among others; Ser70 is shown as reference. Other color references: red, oxygen; blue, nitrogen; green, sulfur. All distances are in angstroms (Å).