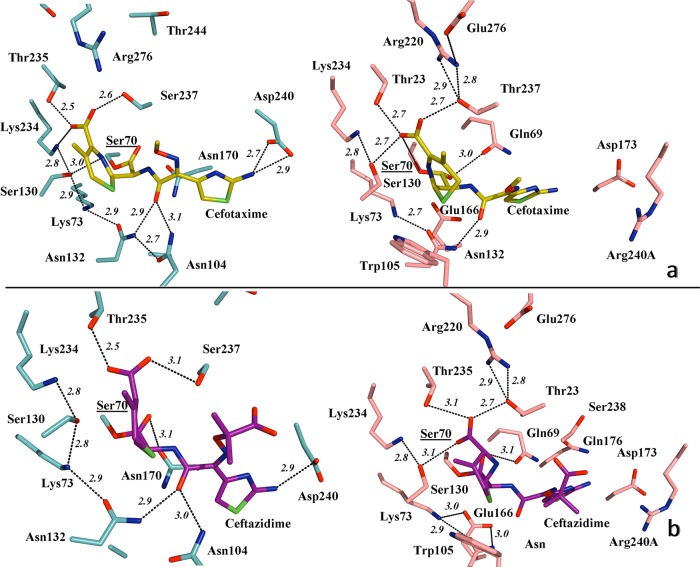
FIG 4.
(a) Detailed view of the active site of TOHO-1 (aqua) in association with cefotaxime (yellow) (left), indicating the main hydrogen bond interactions (PDB 1IYO), and simulated modeling of PER-2 (pink) and the probable positioning of cefotaxime within the active site (right), suggesting the putative most favorable hydrogen bonds and involvement of residues like Gln69, Thr237, and Arg240A in the stabilization of the oxyimino-cephalosporin molecule. (b) Active site of TOHO-1 in complex with acylated ceftazidime (magenta) (left), indicating the main hydrogen bonds (PDB 2ZQD), compared to a simulated model of PER-2 and its probable association with ceftazidime (right), showing the predicted positioning of the molecule and the hydrogen bond interactions (black dashed lines) All distances are in angstroms (Å). Other color references: red, oxygen; blue, nitrogen; green, sulfur. See Results and Discussion for details.