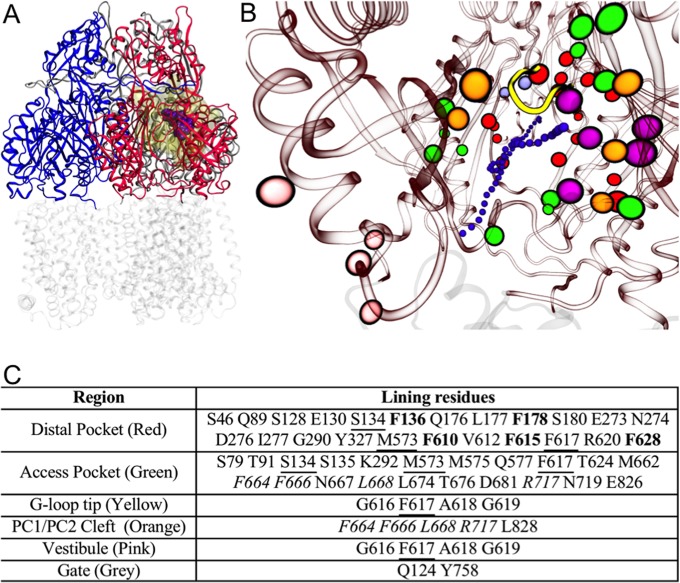
FIG 1.
(A) Reduced model of AcrB used in this work. The transmembrane domain (in transparent gray) was cut off from the protein, and only the periplasmic domain (residues 33 to 335 and 565 to 871 of the intact protein; shown in blue, red, and gray for access, binding, and extrusion protomer, respectively) was kept. The substrate MIN (PDB code 4DX5 [29]) is shown in spheres (violet), and the molecular surface of the residues free to move during the partially restrained MD is shown in transparent yellow. (B) View (perspective looking to the binding protomer) of the key regions of AcrB. The periplasmic and transmembrane domains of AcrB are shown in red and gray transparent ribbons, respectively, while the tip of the G-loop is shown in solid yellow. The residues lining the key regions (see table in panel C) are shown with colored spheres: orange, red, green, magenta, pink, and ice blue for the PC1/PC2 entrance cleft, distal pocket, access pocket, hydrophobic trap, vestibule, and exit gate, respectively. A channel leading from the cleft or the vestibule to the distal pocket is shown by small violet spheres. (C) Table identifying the residues shown in panel B. The residues common to the cleft and the access pocket are italicized, while those shared between the access and distal pockets are underlined. Residues belonging to the hydrophobic trap are in bold red type and colored magenta.