Introduction
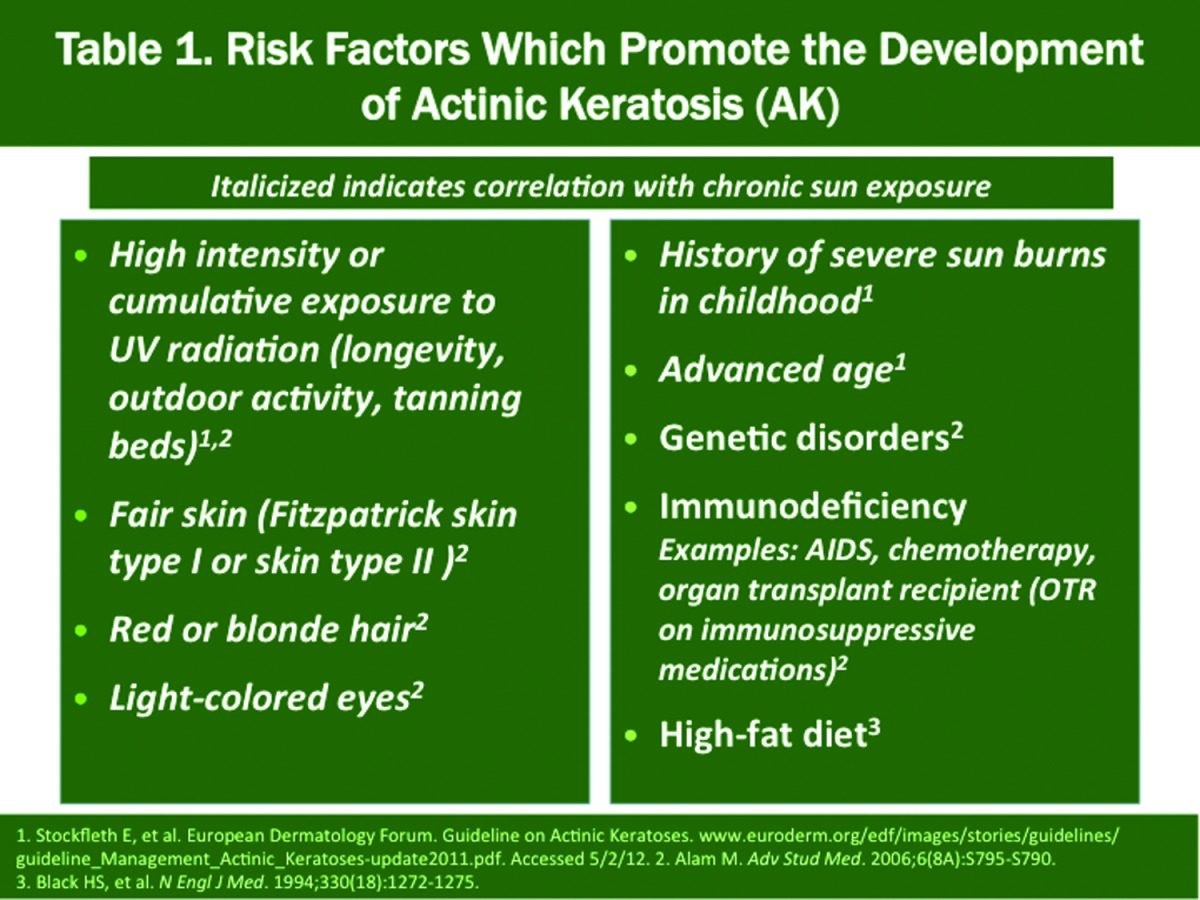
Among the list of chronic cutaneous disorders that are encountered regularly in clinical practice, actinic keratosis (AK) is one of the most common, especially in fair-skinned individuals.1,2 Regardless of whether one defines AK as a premalignant lesion or the first defined stage of squamous cell malignancy, the long-recognized association of AKs with development of nonmelanoma skin cancer (NMSC), especially invasive squamous cell carcinoma (SCC) and squamous cell carcinoma-in-situ (SCC-in-situ), and the high prevalence of AK, dictate that academicians, researchers, and practicing clinicians continue to evaluate how to most effectively manage AK. This includes integrating what is practical, efficient, shown to exhibit both short-term and long-term effectiveness based on the best current evidence, and cost effectiveness. The increasing incidence of NMSC has been directly correlated with the presence of photodamaged skin and the rise in the number of patients with AK seen in clinical practice (Table 1).3 From 1990 to 1999, AK represented 14 percent of all visits to dermatologists in the United States, which totaled 47 million office visits, with AK reported in 2010 to be the most common diagnosis encountered in Caucasian patients seen by dermatologists.1,3,4 Additional data support a rising incidence of NMSC and AK over time.1,5-7
 |
At the present time, the major individual approaches to AK treatment based on available literature and common use in clinical practice are ablative therapy, topical field therapy and photodynamic therapy (PDT).6 Liquid nitrogen cryosurgery is the most frequently used ablative approach for AK treatment. Four topical agents are approved by the United States Food and Drug Administration (FDA) for the treatment of AK (see below). PDT incorporates the use of a specific topically applied photosensitizing agent coupled with subsequent exposure to a designated light source, with FDA approval established with specific photosensitizers and light source application (see below). The following further defines these major therapeutic approaches.
Ablative therapy
Treats only the individual clinically evident AKs where ablative agent is applied;
The most common ablative approach is cryosurgery with liquid nitrogen the most common cryogen used;
Other physical ablative approaches include curettage or tangential (saucerization) excision (often used for hypertrophic AK with specimen sent to pathology to determine if invasive SCC is present).
Topical field therapy
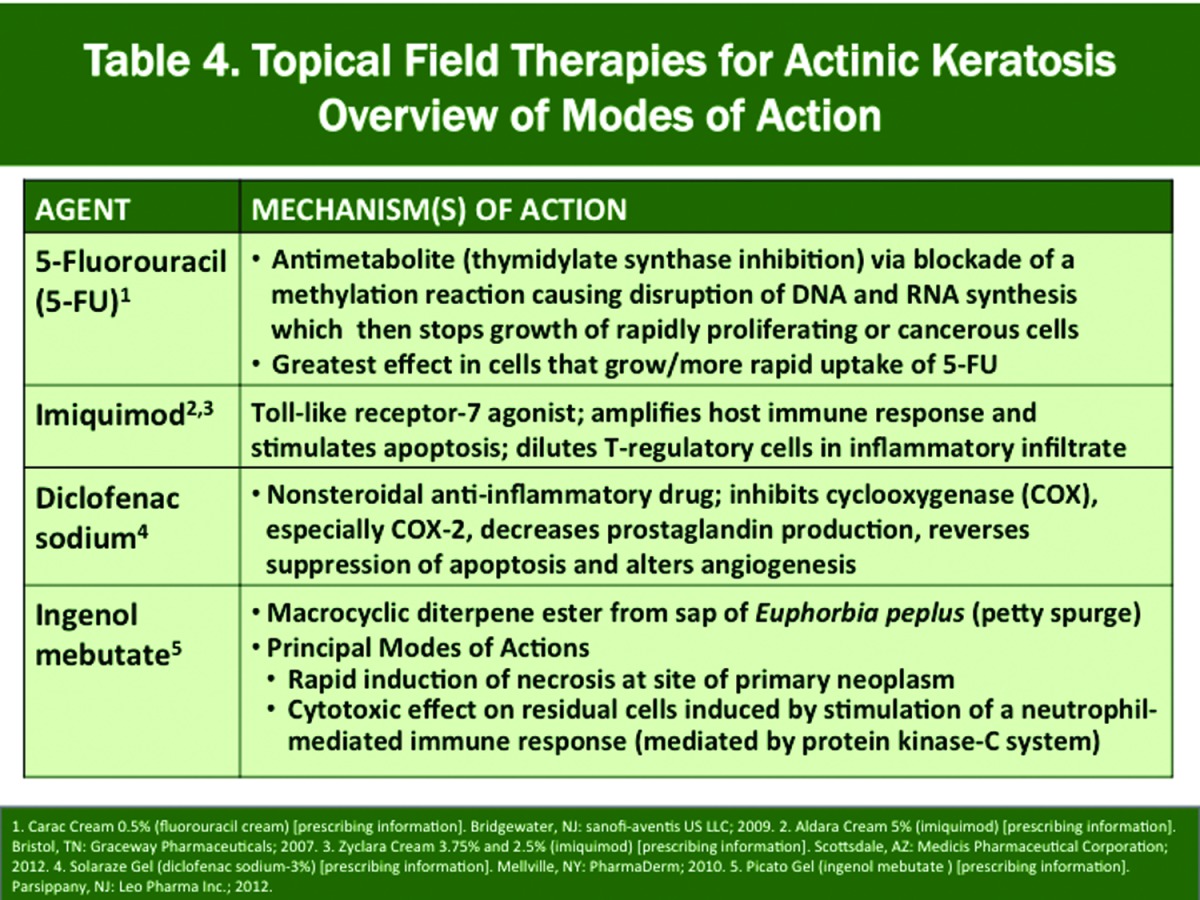
Treats both clinically evident and subclinical (not visible or palpable) AKs in the adjacent field where applied; topical medications approved by the FDA in this group are 5-fluorouracil (5-FU), imiquimod, diclofenac, and ingenol mebutate.
Photodynamic therapy
Incorporates a photosensitizing agent and subsequent exposure to an appropriate light-based energy source;
Can be used as either ablative therapy or as field therapy;
The most common approach is 5-aminolevulinic acid (ALA) and blue light (ALA-PDT); light sources other than blue light have been used although not approved;
Methylaminolevulinic acid (methyl-ALA) has also been used.
The primary educational objectives of this supplement are to:
1) Provide up-to-date information about the “real world” significance of AKs to patients, clinicians, and the healthcare community;
2) Review salient data from studies evaluating approaches to AK treatment;
3) Define what may translate into practical management of AKs in “real world” clinical practice;
4) Define the concept of “field cancerization” and how it relates to assessment and care of the patient with AKs;
5) Outline the limitations of clinical trials and how this relates to the clinician when selecting therapy and providing practical and effective recommendations on use of a given therapy;
6) Review obstacles to the use of topical field therapy and how to mitigate them in “real world” clinical practice;
7) Discuss management approaches that are likely to optimize therapeutic results. It is assumed that the reader has basic knowledge about AKs, including their clinical appearance, histological patterns, and overall clinical relevance.
Actinic Keratosis in “Normal” and Immunocompromised Populations
General population. The diagnosis of AK is highly relevant clinically due to their widespread prevalence, their association with contiguous development of SCC in situ and invasive SCC, and their importance as visible and/or palpable evidence that advanced UV-induced genetic and epigenetic subclinical changes have occurred within the adjacent field of skin that predispose to increased risk of NMSC.3,4,6,8-11 Epidemiological data from 2004 estimated that 58 million people living in the United States would experience more than one AK during a calendar year, with approximately 55 percent of these patients less than 65 years of age.3 The estimated overall prevalence rate of AK in the United States reported in 2006 was 19.65 cases per 100 individuals, with an age-adjusted prevalence rate of 6.5 percent among the general population, and 55.4 percent among men with high chronic sun exposure between 65 and 74 years of age.3 This overall prevalence rate of 6.5 percent in the United States appears to be low as compared to Austria, which was recently reported to be 31.2 percent; however, the methodology used to capture the data was limited to a small fraction of the total population (N=4,449) and included only patients over 30 years of age.7 Nevertheless, these more recent data from Austria were consistent with previous data from the United States and other countries, which showed a higher AK rate in men (39.2% vs. 24.3%) and a rise in incidence of AK with increasing age in both genders, consistent with previous data from the United States and other countries. 1,3~7 Other data from the United States report an overall prevalence rate of 11 to 26 percent.8 In Australia, the AK rate in men and women 30 to 70 years of age were reported to be 55 and 37 percent, respectively9
Immunocompromised population. As the average lifespan continues to get longer, with the incidence of AK continuing to increase with advancing age, and as the presence of AK serves as both a marker and precursor for development of NMSC often within as little as two years (including invasive SCC), the importance of both chronic photodamage and AK as cutaneous diseases is further magnified from both medical and financial perspectives.3,6,8,10,11 Importantly, the increased number of immunocompromised organ transplant recipients (OTR) maintained chronically on immunosuppressive agents, especially those affected by chronic photodamage and AK, is of major significance as the risk for development of invasive SCC has been reported to be 40 percent in this subpopulation as compared to the range of 0.25 to 20 percent per lesion per year reported for the general population.3,10 Importantly, OTRs, especially those with chronically photodamaged skin, often develop a greater number of AKs and cutaneous SCCs over time; the SCC lesions in OTRs often exhibit a larger tumor burden, which increases the potential for metastasis.12,13 When compared to immunocompetent patients, immunocompromised individuals are at a 65-fold to 250-fold higher risk of developing AKs and invasive SCCs.14
Impact of Actinic Keratosis Management on the Health Care Community
Economic impact. The high economic cost associated with treatment of AKs places both this diagnosis and its management under the microscope of both government-related and private third party payors as continued attempts are made to curtail the rising cost of healthcare in the United States. Based on data reported in 2004, total direct cost for AK is estimated at $1.2 billion.3 It has been noted that treatment of AK lesions is completed predominantly in outpatient physician offices. In 2002, approximately 8.2 million office visits were completed for AK with an associated cost of $1 billion, accounting for 92 percent of the total direct cost of AK treatment. According to prescription (Rx) cost data compiled by Scott-Levin, topical Rx medications prescribed for AK treatment accounted for $60 million or five percent of the total cost for AK.3 These data suggest that topical Rx medications for AK, which treat both clinical AKs (visible and palpable) and subclinical AKs (invisible and not palpable) in the adjacent photodamaged field (field treatment), are prescribed overall for a relatively small fraction of patients who are treated for AKs. In fact, the majority of AK treatment administered is as ablative therapy of clinical AKs using liquid nitrogen cryosurgery.3,4,6
Field cancerization concept and its impact on management approaches. The concept of field cancerization was first described in 1953 to describe histological changes in oral mucosal epithelium adjacent to oral SCC.15 Slightly more than one decade later, this concept was applied to AKs as an explanation of why some recurrences occur shortly after completion of treatment.16 This served as an initial support for the concept of field treatment using a topical agent to treat subclinical AKs in addition to clinical AKs. This occurred first with topical 5-FU, which was approved by the FDA in 1970 for the treatment of AKs.6,17 The FDA later approved a different vehicle formulation and strengths of 5-FU (0.5% microsphere cream [2001]).
Three other topical agents received FDA approval for AK over the past 14 years, including diclofenac gel (3% [2000]), imiquimod cream (5% [2004]), 3.75% [2010], 2.5% [2011]), and most recently ingenol mebutate gel (0.015%, 0.05% [2012]), each with different proposed mechanisms of action (MOA), thus allowing clinicians the option to incorporate topical therapy (including field treatment) into the management of AK, either alone or in combination with a physical modality, such as cryosurgery.6,10,17-19 The FDA approval in 1999 of PDT with 5-aminolevulinic acid (ALA), followed by controlled blue light exposure (10J/cm2 over 16 minutes 40 seconds) for treatment of AKs provided another viable option with a unique MOA, with additional studies showing that PDT can be used as a field treatment and with shorter durations of ALA incubation after its application.19-22 Thus, over the past 15 years, more emphasis on the clinical relevance of field therapy for AKs has broadened the vantage point of AK management, with data suggesting that it is most often considered and utilized in patients with diffuse AKs and frequent occurrences of multiple AKs.4,16-18,20,21
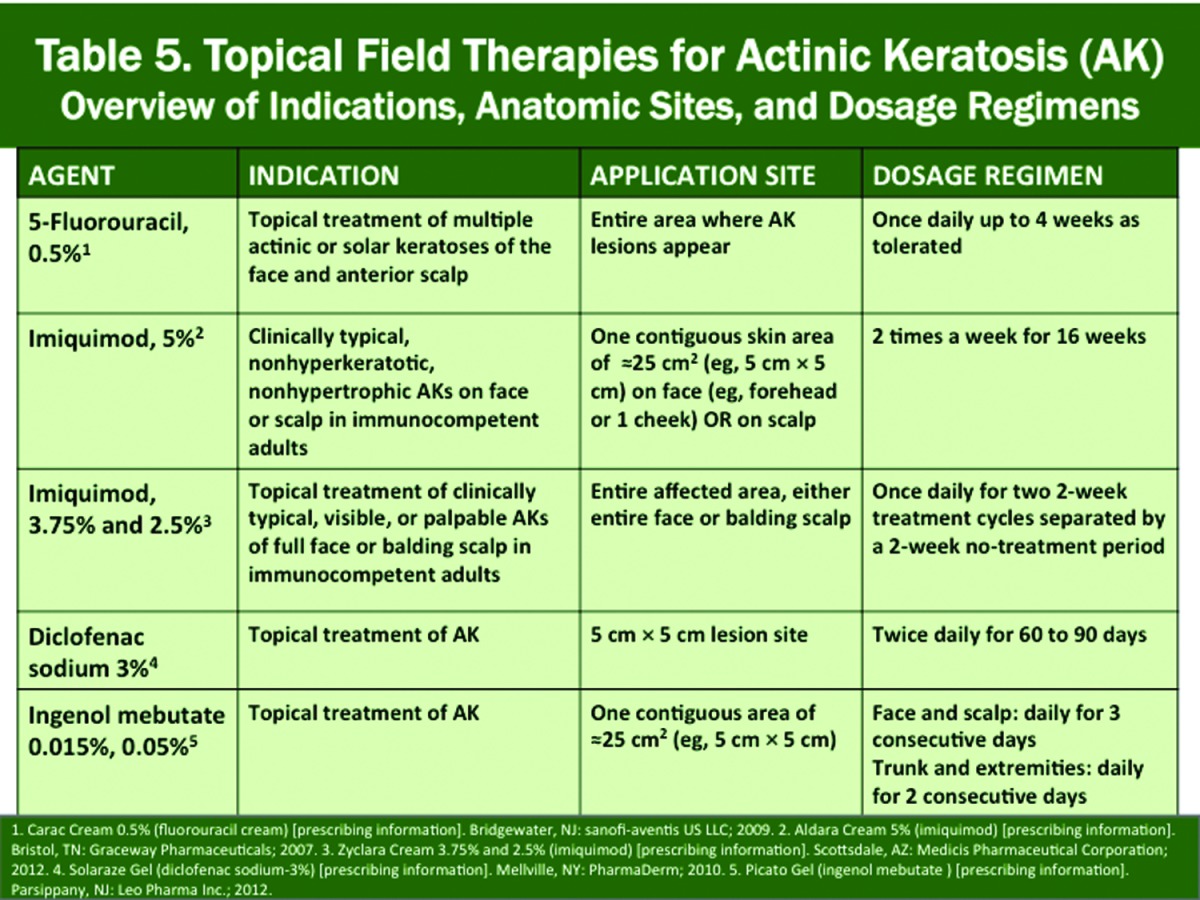
In the FDA-approved product labeling for the different brands of topical medications and for ALA-PDT, differences exist based on the study protocols used in the pivotal trials for AKs that were submitted for approval, including application frequency, duration of therapy, and indicated anatomic sites. In the pivotal studies with all of the more recently approved topical products, efficacy was evaluated for a designated numerical range of non-hyperkeratotic AKs present in a 5x5cm target area with the exception of imiquimod 3.75% and 2.5% creams, which assessed response within an anatomic field (face, balding scalp) affected by a required number of AKs.22-28 When a generic formulation of a specific brand is available, its approved product labeling mirrors that of the original brand product, although the formulations are often not identical in their vehicle composition.
Interestingly, despite the field cancerization concept explaining the strong association of AK with development of NMSC (especially SCC) and the availability since 1970 of field therapies which treat both clinical and subclinical AKs, available data show that field therapy with topical medications for AKs remains underutilized in clinical practice. A retrospective analysis of patients treated for AK (N= 1,793) showed that ablative therapy alone (cryosurgery) was used in approximately 75 percent of visits, with 16 percent and less than 10 percent treated with pharmacological therapy (topical field therapy) alone or a combination of both, respectively21 There are many potential factors that may limit the use of field therapy approaches for AK, which are reviewed in more detail below.
Perspectives on the need to treat actinic keratoses. Previously described as a premalignant skin lesion, AK is now defined as the earliest clinical stage in a continuum of malignancy that may progress to SCC-in-situ and/or invasive SCC, and also serves as an important biological marker for field cancerization.3,4,6,8,10,11,18,29-32
The importance of clinical recognition of AKs and their association with emergence of NMSC was evaluated further in a study that followed 7,784 AKs on the face and ears of 169 patients every six months over six years with careful tracking of the AKs that were clinically diagnosed throughout the study. The risk of progression of AK to primary SCC (invasive or in situ) at one year and five years was 0.60% and 3.4%, respectively33 Approximately two-thirds of primary SCCs and one-third of primary BCCs diagnosed in this study population arose in lesions that were previously diagnosed as AKs, with a 5.6-percent risk of progression to SCC or BCC at five years. Importantly, invasive SCC developed in 2.5 percent of previously diagnosed AKs at five years. An interesting observation was that many previously identified AKs cleared over time without treatment (70% at 5 years); however, no specific clinical features were identified to help predict which AKs would progress to a NMSC and which would resolve spontaneously33
The observation that many AKs resolve spontaneously has led some to question the need to treat AKs.9,33,34 In fact, although spontaneous complete field regression rates are reported to range from 0 to 21 percent, the rate of AK recurrence is 57 percent, with rates of regression of single AK lesions ranging between 15 and 63 percent after one year.35 Thus, spontaneous regression of AKs does not assure that previous or new AKs will not emerge over time. This is based on data evaluating AK subjects from 24 eligible studies that showed a heterogenous spectrum of relative changes in total AK counts over time ranging from -53 to +99.1 percent.35 In addition, relapse of AKs after treatment has also been reported. After previous treatment of AKs primarily with cryosurgery or topical therapy, relapse was observed more commonly in men than women (32 vs. 16%) and with hyperkeratotic AKs as compared to nonhyperkeratotic AKs (33 vs. 21%).36 However, it is not reasonable to expect that therapy of vessentially any skin disorder would always provide complete clearance, especially with a disorder such as AK that is associated with genetic mutations and altered expression of specific biomarkers that promote progression of carcinogenesis.6,,30,37,38 To add, spontaneous resolution of some AKs is an expected phenomenon as deoxyribonucleic acid (DNA) mutations and other abnormalities are averted by inherent self-repair and immunosurveillance mechanisms.6,38,39
As many cases of NMSC develop at anatomic foci of previous AK, and as 60 to 80 percent of invasive SCCs emerge within an AK, the diagnosis of AK indicates the presence of an important disease state of the skin that warrants directed attention, consideration of available options, and implementation of treatment strategies along with periodic follow-up.4 The presence of AK indicates the potential to develop skin malignancy that in some cases is associated with significant morbidity and/or mortality, the latter more often associated with invasive SCC that is aggressive histologically, and/or at anatomic sites, such as the lips, hand, and ear.4,14,18 Therefore, despite the argument that many AKs can resolve spontaneously, it is not known which subclinical AKs will resolve, which will later recur, and which will progress to clinical AK and/or NMSC including invasive SCC, thus supporting a comprehensive management approach that treats both what can be seen or felt (clinical AKs) and what lies beneath (subclinical AKs) (Table 2), the latter through use of field therapy. An optimal comprehensive therapeutic approach for AKs includes three fundamental components
1. Reduce additional genetic mutations that promote AK formation and carcinogenesis through photoprotection methods;
2. Eradicate the clinical AKs that are currently present (ablative and/or field therapy);
3. Treat subclinical AKs that may emerge, but are not currently detectable (field therapy).4,6,10,11,20,29,30,40-47
 |
Management Options for Actinic Keratosis
A complete review of therapeutic options for AK is beyond the scope of this article with applications and outcomes reviewed elsewhere; however, a summary of the major options is provided in Table 3, which includes liquid nitrogen cryosurgery, ALA-PDT, and the four FDA-approved topical therapies. Each therapy exhibits its own MOA and is supported by multiple studies including 1) as monotherapy demonstrating both short-term and long-term AK clearance rates in adult subjects and 2) with use in combination with other modalities, such as cryosurgery.6,10,17-20,22-28,47-72 The modes of action of the four FDA-approved topical field therapies and their approved dosing regimens and application sites are depicted in Tables 4 and 5; however, use as field therapy in clinical practice warrants application to the desired field(s) based on the judgment of the clinician.
TABLE 3.
Major therapeutic options used for the treatment of actinic keratosis (AK)
| THERAPY | COMMENTS |
|---|---|
| Cryosurgery (liquid nitrogen)6,17,48,49 |
Ablative approach:the most common therapy used for AK treatment. MOA: cryonecrosis of epidermis affected by AK Efficacy data: 67% AK clearance rate at 3 months follow up; 57% reduction in number of facial and forearm AKs at 10 months follow up with treatment at baseline and Day 120 along with sunscreen SPF 30. Advantages: efficiency; cost-effective; combination data available with all four FDA-approved topical agents Disadvantages: methodology of use not standardized; limited efficacy data; does not treat subclinical AKs; discomfort (pain); visible erythema, edema, blistering; persistent skin dyschromia. |
| Photodynamic therapy6,17,50-55 |
MOA: 20% ALA converted to protoporphyrin IX (photosensitizer) » controlled exposure to designated light source (i.e., blue light) induces a photodynamic reaction » produces singlet oxygen (damaging to mitochondrial and plasma membranes). FDA approval: AKs on face or scalp using 20% ALA solution and controlled exposure to blue light (10J/cm6 over 16 hrs 40 mins). Advantages: supervised administration; may be used as ablative or field treatment; secondary photorejuvenation effect; short incubation after ALA application shown to be effective (as short as 1 hour); efficacy demonstrated for AKs on upper extremities (efficacy further enhanced by occlusion); adaptable to other light-based devices other than blue light. Disdavantages: photosensitization requires period of strict sunlight avoidance; discomfort; two treatment sessions sometimes needed to optimize initial AK clearance; visible inflammatory reaction may result in some days of “down time.” |
| 5-fluorouracil cream6,17,55-59 |
MOA: anti-metabolite; cytodestructive to rapidly proliferating/neoplastic cells. FDA approval: multiple AKs on the face and anterior scalp applied once daily for up to 4 weeks based on more stringent efficacy data with 0.5% cream (microsphere formulation) than with prior formulations (5%) Advantages: long track record of 5-FU use for AK as a field therapy; efficacy data shows 4 wks > 2 wks >1 wk; combination data with cryotherapy available; limited long-term data on AKs. Disdavantages: greater efficacy requires 2-4 weeks of use; several days to weeks of “down time” due to predictable inflammatory reaction that is visible, often brisk, unsightly, and uncomfortable; marked symptomatology very common (discomfort, pain); long-term data with 5-FU shows less substantivity for clearance of AKs in treated field than imiquimod at 12 months (comparative study). |
| Diclofenac gel6,17,60-63 |
MOA: inhibits primarily cyclo-oxygenase-2 (COX-2), which is upregulated in AK and SCC; COX-2 inhibition reverses suppression of apoptosis. FDA approval: topical treatment of actinic keratosis; applied twice daily for 60 to 90 days. Efficacy data: 90 days > 60 days > 30 days. Combination therapy with cryosurgery available; substantivity of AK reduction at 12 months studied with some benefit. Advantages: Less visible skin inflammation during course of therapy; usually with no “down time”; symptomatology usually negligible. Disdavantages: long course of therapy; twice-daily administration. Some data suggesting lessened risk of SCC emergence in organ transplant recipients (2 years of follow up; longer durations/repeated courses of therapy often needed). |
| Imiquimod cream6,17,64-71 | Cream formulation; initially as 5% cream and later added 3.75% and 2.5% formulations. MOA: Toll-like receptor-7 agonist; induces a directed immune response which promotes mix of cytokines and inflammatory cells that induce apoptosis. Use associated with visible inflammatory response that may range from mild to very brisk; marked visible erythema/crusting is common; symptomatology usually mild to moderate (generally less discomfort than with 5-FU). Rest periods used when inflammatory response very brisk or too symptomatic. FDA approval [5%]: twice a week for 16 weeks for AKs of face or scalp. Several other regimens used with shorter durations, especially cycle therapy (3 weeks on, 3 weeks off, 1 or 2 cycles). FDA approval[3.75%][2.5%]: once daily for 2 weeks, off 2 weeks, once daily for 2 more weeks (2-2-2 regimen) for AKs of full face or balding scalp. Compression of AK therapy over a shorter duration as compared to 5% used as approved or with cycle therapy. Efficacy: 3.75% > 2.5%; both associated with visible inflammatory response. Advantages: efficacy studied in complete fields on face or balding scalp (3.75%, 2.5%); data available for superficial basal cell carcinoma (5%, different treatment regimen); some data for SCC in-situ (5%, case reports); substantivity of AK reduction at 12 months demonstrated (5%, 3.75%); symptomatology associated with visible inflammation is generally mild with lower discomfort overall than with 5-FU. Used with efficacy in organ transplant recipients (longer durations/repeated courses of therapy often needed) Disdavantages: onset and magnitude of visible inflammatory response unpredictable with all concentrations (some very brisk, others moderate or mild); length of “down time” related to visible inflammation; need for rest periods may prolong treatment course; flu-like symptoms in small subset of patients |
| Ingenol mebutate6,17,19,27,72,75-84 | Diterpene ester extracted from the crude sap of the Euphorbia peplus plant; crude sap with long history of medicinal use.FDA approval [0.015%)]: once-daily application for 3 days for AKs on face or scalp. FDA approval [0.05%>]: once daily for 2 days for AKS on the trunk or extremities. MOA: rapid primary necrosis and subsequent immune response (neutrophil-mediated cytotoxicity). Additional comments reviewed in article text. |
 |
 |
Efficacy data from pivotal Phase 3 studies with the FDA-approved agents focus primarily on mean and/or median percentages of complete clearance (100% absence) and partial clearance (>75% reduction) of AKs, with evaluation of these responses restricted within a designated target area.73,74 Other limitations of study enrollment are mandated by the inclusion and exclusion criteria defined in a given study protocol. Thus, it is important that clinicians recognize such limitations and utilize therapies rationally based on each case, as many of the patients encountered in clinical practice have magnitudes of AK involvement and/or associated co-morbidities or concomitant therapies that were excluded in the pivotal clinical trials.72 Examples are the exclusion of
immunocompromised patients and efficacy evaluation only for non-hyperkeratotic AKs. Nevertheless, there is a plethora of information on the overall management of AKs and each of the therapeutic options, including treatment regimens, response rates, local skin reactions, and clinical applications. This provides a good body of available foundation for management decisions, and allows the clinician to extrapolate and apply these therapies using regimens that achieve the therapeutic outcomes needed in each individual patient.
Therapeutic Profile of Ingenol Mebutate for Actinic Keratoses
Ingenol mebutate, derived from the crude sap of the Euphorbia peplus plant, is the newest addition to the armamentarium for treatment of AKs formulated topically applied gel.19,76 Over the past few years, extensive basic science and clinical research have been completed with ingenol mebutate. As the available studies may be less familiar to clinicians due to the more recent availability of ingenol mebutate gel in the United States since 2012, the following provides an overview of data collected on this agent.
Mechanism of action. Dual mechanisms of action have been suggested. Within hours after application, a primary necrosis occurs secondary to mitochondrial swelling; over a period of days, neutrophil-mediated immune response secondary to activation of protein kinase-C induces cytotoxicity of residual proliferating keratinocytes.76-80 In addition, application of ingenol mebutate to murine skin reduced mutant p53 keratinocyte patches by 70 percent after UV exposure.81
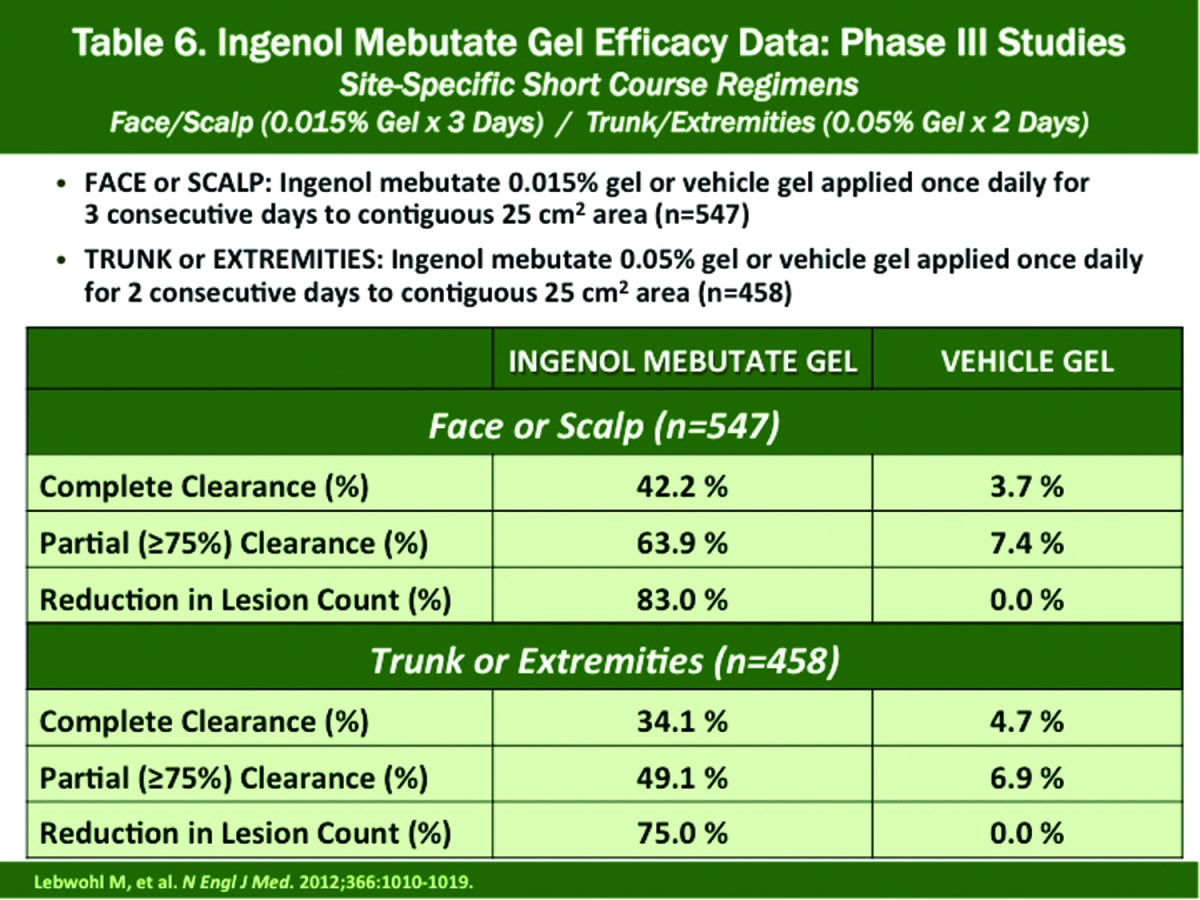
Treatment of Face and Scalp (pivotal Phase 3 studies [n=547]) Efficacy (Table 6). Ingenol mebutate 0.015% applied once daily for three consecutive days produced complete clearance, partial clearance, and lesion reduction rates of 42.2, 63.9, and 83.0 percent, respectively, at Day 57. Corresponding results in vehicle-treated subjects were 3.7, 7.4, and 0.0 percent, respectively19
Treatment of Trunk or Extremities Efficacy (pivotal Phase 3 studies [n=458]) (Table 6). Ingenol mebutate 0.05% applied once daily for two consecutive days produced complete clearance, partial clearance, and lesion reduction rates of 34.1, 49.1, and 75.0 percent, respectively, at Day 57. Corresponding results in vehicle-treated subjects were 4.7, 6.9, and 0.0 percent, respectively19
Tolerability Profile (pivotal Phase 3 studies). Local skin reactions (LSRs) occur after application and include erythema, swelling, vesiculation/ pustulation, crusting, erosion/ulceration, and flaking/scaling.19 Overall, on the face and scalp, the visible inflammation (based on mean composite LSR scores) peaked at Day 4 and resolved by Day 15. On the trunk and extremities, visible inflammation peaks occurred at Day 3 and Day 8, with return almost to baseline by Day 29.19
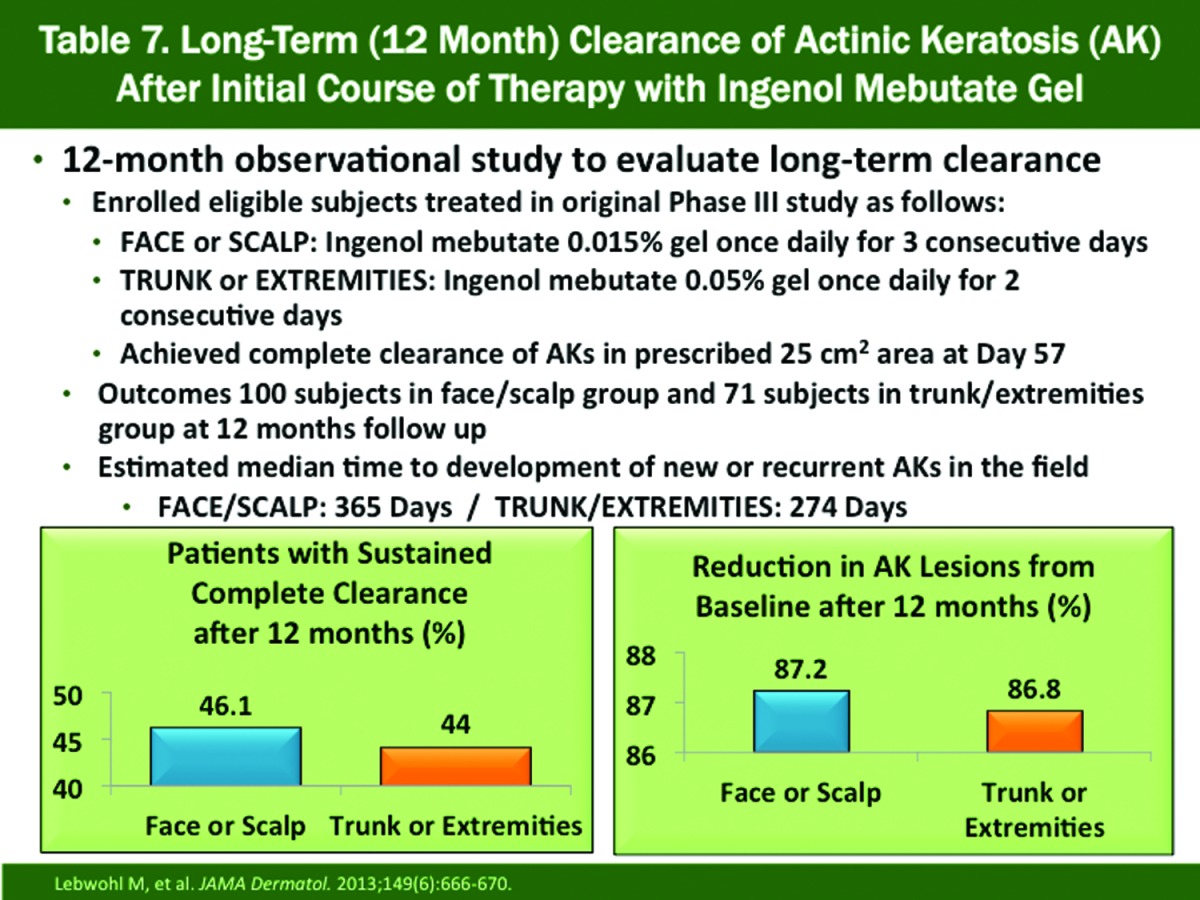
Sustained Clearance at 12 Months (Table 7). This evaluation included subjects with complete clearance of AKs on the face or scalp (n=100) and trunk or extremities (n=71) at Day 57 in pivotal Phase 3 studies and were followed over 12 months; included subjects received no additional AK therapy over the course of the study82 In the face/scalp group, sustained clearance and reduction in AKs from baseline were 46.1 and 87.2 percent, respectively. The corresponding results in the trunk/extremities group were 44 and 86.8 percent, respectively. The estimated median time to recurrence was 365 days on the face or scalp and 274 days on the trunk or extremities.82
Cryosurgery Followed By Ingenol Mebutate 0.015% Gel for Face or Scalp (N=329). In a Phase 3, double-blind, randomized study, subjects with AK on the face or scalp in a designated target area (25cm2) were treated once daily for three days with either ingenol mebutate 0.015% gel or vehicle gel at three weeks after cryosurgery for the baseline AKs. All subjects received no other treatments for AKs over the 12-month course of the study72 The complete clearance rates in the ingenol mebutate-treated arm were 60.6 and 30.5 percent at Week 11 and Month 12, respectively. The corresponding results in the vehicle-treated subjects were 49.4 and 18.5 percent, respectively. These comparative results were statistically significant at Week 11 (P=0.04) and Month 12 (P=0.01). At Month 12, mean percentage reduction of AKs was statistically significantly (P=0.002) greater with ingenol mebutate (68.2%) than with vehicle (54.1%). The likelihood of remaining free of lesions was sustained longer with ingenol mebutate compared with vehicle gel at six months (78% vs. 68%), at nine months (64% vs. 57%), and at 12 months (55% vs. 40%), respectively. Local skin reactions associated with ingenol mebutate all resolved within 14 days on application.72
 |
 |
The body of available data on ingenol mebutate for AKs demonstrates a marked reduction of AKs involving four different anatomic areas with demonstration of sustained clearance in many patients over 12 months when used as monotherapy or in combination with cryosurgery.19,72 Due to the quick onset of action, the duration of visible inflammation (erythema, other LSRs) is much shorter overall than with topical 5-FU and topical imiquimod, especially on the face and scalp; tolerability with this therapy is also favorable overall without marked pain or physical discomfort. Although not FDA approved for BCC, SCC-in-situ, and SCC, there is emerging evidence that ingenol mebutate may be effective in some cases, especially superficial BCC and SCC-in-situ, although poor efficacy for seborrheic keratoses was shown.83,84
Obstacles to the Use of Topical Field Therapy for Actinic Keratosis
Despite the fact that available data support augmented initial and sustained clearance with use of field therapy in conjunction with cryosurgery, there are several potential obstacles that deter clinicians from prescribing field therapy in patients with AKs. These include the increased time associated with explaining the regimen, proper application of the medication, potential visible responses (including associated signs and symptoms of inflammation) and time course of response; working around the “down time” periods of visible inflammation at sites of application; access to medication; cost of medication; and the individual perceptions of clinicians regarding the benefits of field therapy.
Time used to educate patients. Time factors related to education of patients cannot be totally removed as education of patients is a significant component of what clinicians and their staff need to do. However, the education process can be time-efficient by being sure that all involved are fully educated on and consistent with what information needs to be explained. The messages need to be succinct, straightforward, clear, and accurate. It is important for patients to understand why a topical field therapy is being recommended and how it is to be used (if a self-applied medication). If treated with PDT, it is important for the patient to know the duration of time they need to avoid any sun or UV light exposure. Written handout materials can be used to support the verbal explanation.
Treatment-induced visible inflammation. “Down time” related to anticipated visible inflammation induced by a treatment is important to address as most AK treatments are used on regularly exposed skin sites. Visible signs and symptoms of inflammation (i.e., erythema, crusting) are often cosmetically displeasing and can last for days to weeks depending on the therapy used. An advantage of ingenol mebutate therapy is the shorter duration of visible inflammation and associated “down time,” especially on the face and scalp. It is important to address with the patient the available therapeutic choices and their associated visible inflammatory changes with consideration of potential “down time” so that specific therapies can be timed acceptably with the full awareness and consent of the patient.
Cost and access. The cost of medication to the patient and access to specific therapies are very relevant issues that have a direct impact on therapeutic outcomes as they often place a dividing line between selection of therapy by the clinician and completion of therapy by the patient. It is important for the clinician to delegate to specific staff members the responsibility of learning details regarding access to specific therapies and costs to the patient as best as possible, at least with the most predominant third-party payers in their geographic area. Knowing which pharmacies in the geographic area are dedicated to assist patients with available cost savings programs (i.e., rebate systems, Medicare part D details, specialty pharmacy services, etc.) is also very important. Cost and access are “moving targets” and represent major challenges in the treatment of patients with AKs. A successful outcome requires that all parties involved work together on trying to optimize access to the care that is recommended for the patient.
Clinician perspectives of field therapy. Many clinicians become creatures of habit simply because that is human nature, and this may explain in some cases why certain clinicians do not often use topical field therapy. Most prescribe topical field therapy selectively in patients who continue to return with multiple AKs or start to develop NMSCs rather than to consider its potential value earlier in the course of therapy21,74 Concerns about cost and access are also important factors. Lastly, some clinicians may not accept the therapeutic concepts behind field cancerization and field therapy. In this situation, continued educational efforts and additional research may modify the beliefs and practices of some clinicians over time.
Optimizing Therapeutic Outcomes: Putting It All Together
The first step in optimizing therapeutic outcomes is to recognize the significance of AKs as an early clinically evident squamous skin malignancy and as a marker for the increased potential for development of NMSC, including invasive SCC. It is fortunate that clinicians are equipped with a variety of therapeutic options that can be used depending on patient-specific details involved with each case. The following summarizes information presented on the major available therapies for AKs.
Liquid nitrogen cryosurgery is very useful as an ablative therapy to treat clinical AKs. The clearance rate is not 100 percent and follow up is needed. Adverse sequelae are pain, blistering, and prolonged or permanent dyschromia at treatment sites. PDT may be utilized as an ablative option and can also be applied as field therapy.
Topical field therapies are effective in treating both clinical and subclinical AKs. Modes of action and suggested regimens for AKs differ among the available agents. Visible inflammation is common with most of these agents so potential “down time” needs to be addressed up front with the patient. Topical diclofenac produces the least visible inflammation overall, but requires application twice daily over a much longer treatment duration. Ingenol mebutate gel provides a much shorter period of visible inflammation (down time) as compared to topical 5-FU and imiquimod.
The FDA-approved regimens and their corresponding response rates represent the “average” monotherapy results based on a number range of non-hyperkeratotic AKs in a designated area in immunocompetent patients that were included in the studies. In the real world, clinicians see cases that exceed these limitations. Therefore, clinicians must adapt data from studies to what is likely to apply to given patients they encounter in practice. Combination with cryosurgery offers augmented therapeutic benefit, and in some cases repeated courses of field therapy over time may be warranted.
References
- 1.Davis SA, Narahari S, Feldman SR, et al. Top dermatologic conditions in patients of color: an analysis of nationally representative data. J Drugs Dermatol. 2012;ll(4):466–473. [PubMed] [Google Scholar]
- 2.Weinstock MA, Boyle MM. Statistics on interest to the dermatologist: visits to non-federal office-based physicians in the United States, 2009. In: Del Rosso JQ, editor. Year Book of Dermatology and Dermatologic Surgery 2012. Philadelphia: Elsevier-Mosby; 2012. p. 67. [Google Scholar]
- 3.Bickers DR, Lim HW, Margolis D, et al. The burden of skin diseases: 2004 a joint project of the American Academy of Dermatology Association and the Society for Investigative Dermatology. J Am Acad Dermatol. 2006;55:490–500. doi: 10.1016/j.jaad.2006.05.048. [DOI] [PubMed] [Google Scholar]
- 4.Lee AD, Jorizzo JL. Optimizing management of actinic keratosis and photodamaged skin. Utilizing a step-wise approach. Cutis. 2009;84:169–175. [PubMed] [Google Scholar]
- 5.Gupta AK, Cooper EA, Feldman SR. A survey of office visits for actinic keratosis as reported by NAMCS 1990-1999. National Ambulatory Medical Care Survey. Cutis. 2002;70(Suppl2):8–13. [PubMed] [Google Scholar]
- 6.Williams V, Rosen T, Ceilley RI, et al. Topical treatment of skin cancer. In: Rogel DS, Robinson JK, Ross M, et al., editors. Cancer of the Skin, 2nd ed. Philadephia: Elsevier-Saunders; 2011. pp. 462–476. [Google Scholar]
- 7.Eder J, Prillinger K, Korn A, et al. Prevalence of actinic keratosis among dermatology outpatients in Austria. Br J Dermatol. doi: 10.1111/bjd.13132. 2014 May 23. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 8.Salasche S. Epidemiology of actinic keratosis and squamous cell carcinoma. J Am Acad Dermatol. 2000;42:4–7. doi: 10.1067/mjd.2000.103342. [DOI] [PubMed] [Google Scholar]
- 9.Frost C, Williams G, Green A. High incidence and regression rates of solar keratoses in a Queensland community. J Invest Dermatol. 2000;115:273–277. doi: 10.1046/j.1523-1747.2000.00048.x. [DOI] [PubMed] [Google Scholar]
- 10.Ulrich M, Drecoll U, Stockfleth E. Emerging drugs for actinic keratosis. Exp Opin Emerg Drugs. 2010;15(4):545–55. doi: 10.1517/14728214.2010.507191. [DOI] [PubMed] [Google Scholar]
- 11.Fuchs A, Marmur E. The kinetics of skin cancer: progression of actinic keratosis to squamous cell carcinoma. Dermatol Surg. 2007;33:1099–1101. doi: 10.1111/j.1524-4725.2007.33224.x. [DOI] [PubMed] [Google Scholar]
- 12.Forchetti G, Suppa M, Del Marmol V. Overview on non-melanoma skin cancers in solid organ transplant recipients. G Ital Dermatol Venereal, 2014;149(4):383–387. [PubMed] [Google Scholar]
- 13.Zwald FO, Brown M. Skin cancer in solid organ transplant recipients: advances in therapy and management: part I Epidemiology of skin cancer in solid organ transplant recipients. J Am Acad Dermatol. 2011;65(2):253–261. doi: 10.1016/j.jaad.2010.11.062. [DOI] [PubMed] [Google Scholar]
- 14.Euvrard S, Kanitakis J, Claudy A. Skin cancer after organ transplantation. N Eng J Med. 2003;346(17):1681–1691. doi: 10.1056/NEJMra022137. [DOI] [PubMed] [Google Scholar]
- 15.Slaughter DP, Southwick HW, Smejkal W. Field cancerization in oral stratified squamous epithelium; clinical implications of multicentric origin. Cancer. 1953;6(5):963–968. doi: 10.1002/1097-0142(195309)6:5<963::aid-cncr2820060515>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 16.Epstein E. Planing for precancerous skin. A ten-year evaluation. Calif Med. 1966;105(l):26–27. [PMC free article] [PubMed] [Google Scholar]
- 17.Gupta AK, Paquet M. Network meta-analysis of the outcome “participant complete clearance” in nonimmunosuppressed participants of eight interventions for actinic keratosis: a follow-up on a Cochrane review. Br J Dermatol. 2013;169(2):250–259. doi: 10.1111/bjd.12343. [DOI] [PubMed] [Google Scholar]
- 18.Del Rosso JQ. Current regimens and guideline implications for the treatment of actinic keratosis: proceedings of a clinical roundtable at the 2011 Winter Clinical Dermatology Conference. Cutis. 2011;88(Suppl 1[ii]):1–8. [PubMed] [Google Scholar]
- 19.Lebwohl M, Swanson N, Anderson LL, et al. Ingenol mebutate gel for actinic keratosis. N Engl J Med. 2012;366(11):1010–1019. doi: 10.1056/NEJMoa1111170. [DOI] [PubMed] [Google Scholar]
- 20.Stockfleth E. The paradigm shift in treating actinic keratosis: a comprehensive strategy. J Drugs Dermatol. 2012;11(12):1462–1467. [PubMed] [Google Scholar]
- 21.Balkrishnan R, Cayce KA, Kulkarni AS, et al. Predictors of treatment choices and associated outcomes in actinic keratoses: results from a national physician survey study. J Dermatolog Treat. 2006;17(3):162–166. doi: 10.1080/09546630600765081. [DOI] [PubMed] [Google Scholar]
- 22. Carac 0.5% cream [package insert]. Bridgewater, NJ: Valeant Pharmaceuticals; 2006.
- 23. Solaraze 3% gel [package insert]. Princeton, NJ: Sandoz/PharmaDerm; 2008.
- 24. Levulan 5% Kerastick [Package insert]. Wilmington, MA: Dusa; 1999.
- 25. Aldara 5% cream [package insert]. Bridgewater, NJ: Valeant Pharmaceuticals; 2009.
- 26. Zyclara 3.75% cream, 2.5% cream [package insert], Bridgewater, NJ: Valeant Pharmaceuticals; 2010.
- 27. Picato 0.015% gel, 0.05% gel [package insert]. Parsippany, NJ: Leo Pharma Inc; 2009.
- 28.Ibrahim SF, Brown MD. Actinic keratoses: a comprehensive review. J Clin Aesthetic Dermatol. 2009;2:43–48. [PMC free article] [PubMed] [Google Scholar]
- 29.Feldman SR, Fleischer AB. Progression of actinic keratosis to squamous cell carcinoma revisited: clinical and treatment implications. Cutis. 2011 Apr;87(4):201–207. [PubMed] [Google Scholar]
- 30.Vatve M, Ortonne JP, Birch-Machina MA, et al. Management of field change in actinic keratosis. Br J Dermatol. 2007;157(Suppl2):21–24. doi: 10.1111/j.1365-2133.2007.08268.x. [DOI] [PubMed] [Google Scholar]
- 31.Martin DM. Impact of interval and combination therapies on the management of actinic keratosis: review and clinical considerations. J Dermatolog Treat. 2011;22(5):288–297. doi: 10.3109/09546631003797072. [DOI] [PubMed] [Google Scholar]
- 32.Fu W, Cockerell CJ. The actinic (solar) keratosis: a 21st century perspective. Arch Dermatol. 2003;139:66–70. doi: 10.1001/archderm.139.1.66. [DOI] [PubMed] [Google Scholar]
- 33.Criscione VD, Weinstock MA, Naylor M, et al. Actinic keratosis: Natural history and risk of malignant transformation in the Veterans Affairs Topical Tretinoin Chemoprevention Trial. Cancer. 2009;115:2523–2530. doi: 10.1002/cncr.24284. [DOI] [PubMed] [Google Scholar]
- 34.Marks R, Foley P, Goodman G, et al. Spontaneous remission of solar keratoses: the case for conservative management. Br J Dermatol. 1986;115:649–655. doi: 10.1111/j.1365-2133.1986.tb06644.x. [DOI] [PubMed] [Google Scholar]
- 35.Werner RN, Sammain A, Erdmann R, et al. The natural history of actinic keratosis: a systematic review. Br J Dermatol. 2013;169(3):502–518. doi: 10.1111/bjd.12420. [DOI] [PubMed] [Google Scholar]
- 36.Javor S, Chimenti S, Patrizi A, et al. Relapsed actinic keratosis evaluation: an observational Italian multicenter prospective study. Does gender have a role? G Ital Dermatol Venereol, 2014;149(2):199–204. [PubMed] [Google Scholar]
- 37.Filipowicz E, Adegboyega P, Sanchez RL, et al. Expression of CD95 (Fas) in sun-exposed human skin and cutaneous carcinoma. Cancer. 2002;94:814–819. doi: 10.1002/cncr.10277. [DOI] [PubMed] [Google Scholar]
- 38.Ziegler A, Jonason A, Simon J, et al. Tumor suppressor gene mutations and photocarcinogenesis. Photochem Photobiol. 1996;63(4):432–435. doi: 10.1111/j.1751-1097.1996.tb03064.x. [DOI] [PubMed] [Google Scholar]
- 39.Chung J, Cho S, Kang S. Why does the skin age? Intrinsic aging, photoaging, and their pathophysiology. In: Rigel DS, Weiss RA, Lim HW, Dover J, editors. Photoaging. New York: Marcel Dekker Inc.; 2004. pp. 1–13. [Google Scholar]
- 40.Ananthaswamy HN, Loughlin SM, Cox P, et al. Sunlight and skin cancer: inhibition of p53 mutations in UV-irradiated mouse skin by sunscreens. Nat Med. 1997;3(5):510–514. doi: 10.1038/nm0597-510. [DOI] [PubMed] [Google Scholar]
- 41.Goldenberg A, Nguyen BT, Jiang SI. Knowledge, understanding, and use of preventive strategies against non-melanoma skin cancer in healthy and immunosuppressed individuals undergoing Mohs surgery. Dermatol Surg. 2014;40(2):93–100. doi: 10.1111/dsu.12399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stockfleth E, Ferrandiz C, Grob JJ, et al. Development of a treatment algorithm for actinic keratoses: a European Consensus. Eur J Dermatol. 2008;18(6):651–659. doi: 10.1684/ejd.2008.0514. [DOI] [PubMed] [Google Scholar]
- 43.Rossi R, Calzavara-Pinton PG, Giannetti A, et al. Italian guidelines and therapeutic algorithm for actinic keratoses. G Ital Dermatol Venereol. 2009;144(6):713–723. [PubMed] [Google Scholar]
- 44.Stockfleth E, Gupta G, Peris K, et al. Reduction in lesions from Lmax: a new concept for assessing efficacy of field-directed therapy for actinic keratosis, results with imiquimod 3.75% Eur J Dermatol. 2014;24(l):23–27. doi: 10.1684/ejd.2014.2265. [DOI] [PubMed] [Google Scholar]
- 45.Dodds A, Chia A, Shumack S. Actinic keratosis: rationale and management. Dermatol Ther (Heidelb). 2014;4(1):11–31. doi: 10.1007/s13555-014-0049-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Berman B, Cockerell CJ. Pathobiology of actinic keratosis: ultraviolet-dependent keratinocyte proliferation. J Am Acad Dermatol. 2013;68(1 Suppl 1):S10–S19. doi: 10.1016/j.jaad.2012.09.053. [DOI] [PubMed] [Google Scholar]
- 47.Berman B, Cohen DE, Amini S. What is the role of field-directed therapy in the treatment of actinic keratosis? Part 2: Commonly used field-directed and lesion-directed therapies. Cutis. 2012;89(6):294–301. [PubMed] [Google Scholar]
- 48.Thai KE, Fergin P, Freeman M, et al. A prospective study of the use of cryosurgery for the treatment of actinic keratoses. Int J Dermatol. 2004;43(9):687–692. doi: 10.1111/j.1365-4632.2004.02056.x. [DOI] [PubMed] [Google Scholar]
- 49.Ianhez M, Miot HA, Bagatin E. Liquid nitrogen for the treatment of actinic keratosis: a longitudinal assessment. Gryobiology. doi: 10.1016/j.cryobiol.2014.06.006. 2014 Jun 30. [DOI] [PubMed] [Google Scholar]
- 50.Jeffes EW, McCullough JL, Weinstein GD, et al. Photodynamic therapy of actinic keratosis with topical 5-aminolevulinic acid. A pilot dose-ranging study. Arch Dermatol. 1997;133(6):727–732. [PubMed] [Google Scholar]
- 51.Fowler JF, Jr, Zax RH. Aminolevulinic acid hydrochloride with photodynamic therapy: efficacy outcomes and recurrence 4 years after treatment. Cutis. 2002;69(suppI6):2–7. [PubMed] [Google Scholar]
- 52.Wan MT, Lin JY. Current evidence and applications of photodynamic therapy in dermatology. Clin Gosmet Investig Dermatol. 2014;7:145–163. doi: 10.2147/CCID.S35334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Touma D, Yaar M, Whitehead S, et al. A trial of short incubation, broad-area photodynamic therapy for facial actinic keratoses and diffuse photodamage. Arch Dermatol. 2004;140(1):33–40. doi: 10.1001/archderm.140.1.33. [DOI] [PubMed] [Google Scholar]
- 54.Haddad A, Santos ID, Gragnani A, et al. The effect of increasing fluence on the treatment of actinic keratosis and photodamage by photodynamic therapy with 5-aminolevulinic acid and intense pulsed light. Photomed Laser Surg. 2011;29(6):427–432. doi: 10.1089/pho.2009.2733. [DOI] [PubMed] [Google Scholar]
- 55.Martin G. Prospective, case-based assessment of sequential therapy with topical fluorouracil cream 0.5% and ALA-PDT for the treatment of actinic keratosis. J Drugs Dermatol. 2011;10(4):372–378. [PubMed] [Google Scholar]
- 56.Weiss J, Menter A, Hevia O, et al. Effective treatment of actinic keratosis with 0.5% fluorouracil cream for 1, 2, or 4 weeks. Cutis. 2002;70(2 Suppl):22–29. [PubMed] [Google Scholar]
- 57.Gupta AK. The management of actinic keratoses in the United States with topical fluorouracil: a pharmacoeconomic evaluation. Cutis. 2002;70:30–36. [PubMed] [Google Scholar]
- 58.Jorizzo J, Weiss J, Furst K, et al. Effect of a 1-week treatment with 0.5% topical fluorouracil on occurrence of actinic keratosis after cryosurgery: a randomized, vehicle-controlled clinical trial. Arch Dermatol. 2004;140(7):813–816. doi: 10.1001/archderm.140.7.813. [DOI] [PubMed] [Google Scholar]
- 59.Stough D, Bucko AD, Vamvakias G, et al. Fluorouracil cream 0.5% for actinic keratoses on multiple body sites: an 18-month open-label study. Cutis. 2010;85(5):267–273. [PubMed] [Google Scholar]
- 60.Wolf JE, Jr, Taylor JR, Tschen E, et al. Topical 3.0% diclofenac in 2.5% hyaluronan gel in the treatment of actinic keratoses. Int J Dermatol. 2001;40(11):709–713. doi: 10.1046/j.1365-4362.2001.01324.x. [DOI] [PubMed] [Google Scholar]
- 61.Nelson C, Rigel D. Long-term follow up of diclofenac sodium 3% in 2.5% hyaluronic acid gel for actinic keratosis: one-year evaluation. J Clin Aesthet Dermatol. 2009;2(7):20–25. [PMC free article] [PubMed] [Google Scholar]
- 62.Martin GM, Stockfleth E. Diclofenac sodium 3% gel for the management of actinic keratosis: 10+ years of cumulative evidence of efficacy and safety. J Drugs Dermatol. 2012;11(5):600–608. [PubMed] [Google Scholar]
- 63.Ulrich C, Johannsen A, Rowert-Huber J, et al. Results of a randomized, placebo-controlled safety and efficacy study of topical diclofenac 3% gel in organ transplant patients with multiple actinic keratoses. Eur J Dermatol. 2010;20(4):482–488. doi: 10.1684/ejd.2010.1010. [DOI] [PubMed] [Google Scholar]
- 64.Miller RL, Gerster JF, Owens ML, et al. Imiquimod applied topically: a novel immune response modifier and new class of drug. Int J Immunopharmacol. 1999;21(1):1–14. doi: 10.1016/s0192-0561(98)00068-x. [DOI] [PubMed] [Google Scholar]
- 65.Ooi T, Barnetson RS, Zhuang L, et al. Imiquimod-induced regression of actinic keratosis is associated with infiltration by T lymphocytes and dendritic cells: a randomized controUed trial. Br J Dermatol. 2006;154(1):72–78. doi: 10.1111/j.1365-2133.2005.06932.x. [DOI] [PubMed] [Google Scholar]
- 66.Torres A, Storey L, Anders M, et al. Immune-mediated changes in actinic keratosis following topical treatment with imiquimod 5% cream. J Transl Med. 2007;26:5–7. doi: 10.1186/1479-5876-5-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Del Rosso JQ. The use of topical imiquimod for the treatment of actinic keratosis: a status report. Cutis. 2005;76(4):241–248. [PubMed] [Google Scholar]
- 68.Krawtchenko N, Roewert-Huber J, Ulrich M, et al. A randomized study of topical 5% imiquimod vs topical 5- fluorouracil vs cryosurgery in immunocompetent patients with actinic keratoses: a comparison of clinical and histological outcomes including I-year follow-up. Br J Dermatol. 2007;157(Suppl2):34–40. doi: 10.1111/j.1365-2133.2007.08271.x. [DOI] [PubMed] [Google Scholar]
- 69.Swanson N, Abramovits W, Berman B, et al. Imiquimod 2.5% and 3.75% for the treatment of actinic keratoses: results of two placebo-controlled studies of daily application to the face and balding scalp for two 2-week cycles. J Am Acad Dermatol. 2010;62(4):582–590. doi: 10.1016/j.jaad.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 70.Hanke CW, Swanson N, Bruce S. Complete clearance is sustained for at least 12 months after treatment of actinic keratoses of the face or balding scalp via daily dosing with imiquimod 3.75% or 2.5% cream. J Drugs Dermatol. 2011;10(2):165–170. [PubMed] [Google Scholar]
- 71.Del Rosso JQ, Sofen H, Leshin B, et al. Safety and efficacy of multiple 16-week courses of topical imiquimod for the treatment of large areas of skin involved with actinic keratoses. J Clin Aesthet Dermatol. 2009;2(4):20–28. [PMC free article] [PubMed] [Google Scholar]
- 72.Berman B, Goldenberg G, Hanke CW, et al. Efficacy and safety of ingenol mebutate 0.015% gel after cryosurgery of actinic keratosis: 12-month results. J Drugs Dermatol. 2014;13(6):741–747. [PubMed] [Google Scholar]
- 73.Wolf JE, Jr, Rigel DS. Understanding efficacy end-points in studies of field-directed therapy for actinic keratosis. Int J Dermatol. 2013;52(9):1063–1070. doi: 10.1111/j.1365-4632.2012.05776.x. [DOI] [PubMed] [Google Scholar]
- 74.Hagele TJ, Levender MM, Davis SA, et al. Practice trends in the treatment of actinic keratosis in the United States: 0.5% fluorouracil and combination cryotherapy plus fluorouracil are underused despite evidence of benefit. J Gutan Med Surg. 2012;16(2):107–114. doi: 10.2310/7150.2011.11002. [DOI] [PubMed] [Google Scholar]
- 75.Ogbourne SM, Suhrbier A, Jones B, et al. Antitumor activity of 3-ingenyl angelate: plasma membrane and mitochondrial disruption and necrotic cell death. Cancer Res. 2004;64(8):2833–2839. doi: 10.1158/0008-5472.can-03-2837. [DOI] [PubMed] [Google Scholar]
- 76.Rosen RH, Gupta AK, Tyring SK. Dual mechanism of action of ingenol mebutate gel for topical treatment of actinic keratoses: Rapid lesion necrosis followed by lesion- specific immune response. J Am Acad Dermatol. 2012;66(3):486–493. doi: 10.1016/j.jaad.2010.12.038. [DOI] [PubMed] [Google Scholar]
- 77.Kedei N, Lundberg DJ, Toth A, et al. Characterization of the interaction of ingenol 3-angelate with protein kinase C. Cancer Res. 2004;64:3243–3255. doi: 10.1158/0008-5472.can-03-3403. [DOI] [PubMed] [Google Scholar]
- 78.Challacombe JM, Suhrbier A, Parsons PG, et al. Neutrophils are a key component of the antitumor efficacy of topical chemotherapy with ingenol-3-angelate. J Immunol. 2006;177:8123–8132. doi: 10.4049/jimmunol.177.11.8123. [DOI] [PubMed] [Google Scholar]
- 79.Hampson P, Chahal H, Khanim F, et al. PEP005, a selective small-molecule activator of protein kinase C has potent antileukemic activity mediated via the delta isoform of PKC. Blood. 2005;106(4):1362–1368. doi: 10.1182/blood-2004-10-4117. [DOI] [PubMed] [Google Scholar]
- 80.Stahlhut M, Lord JM, Bertelsen M, et al. Ingenol mebutate initiates multiple specific cell death pathways in human cancer cells. J Am Acad Dermatol. 2012;66(4 Suppl 1) AB152. Abstract presented at the 70th Annual Meeting of the American Academy of Dermatology; March 16 20, 2012; San Diego, California. [Google Scholar]
- 81.Cozzi SJ, Ogbourne SM, James C. Ingenol mebutate field-directed treatment of UVB-damaged skin reduces lesion formation and removes mutant p53 patches. J Invest Dermatol. 2012;132(4):1263–1271. doi: 10.1038/jid.2011.418. [DOI] [PubMed] [Google Scholar]
- 82.Lebwohl M, Shumack S, Stein Gold L, et al. Long-term follow-up study of ingenol mebutate gel for the treatment of actinic keratoses. JAMA Dermatol. 2013;149(6):666–670. doi: 10.1001/jamadermatol.2013.2766. [DOI] [PubMed] [Google Scholar]
- 83.Ghuznavi G, Nocera NF, Guajardo AR, et al. Emerging medical treatments for actinic keratoses, squamous cell carcinoma and basal cell carcinoma. Clin Invest. 2012;2(9):909–921. [Google Scholar]
- 84.Freeman M, Rosen R, Zibert JR, et al. Ingenol mebutate gel topically applied under occlusion to superficial basal cell carcinoma is efficacious compared with marginal effect in seborrheic keratosis (poster 7934). Poster presented at the 72nd Annual American Academy of Dermatology Meeting; March 21-25, 2014; Denver, Colorado.


