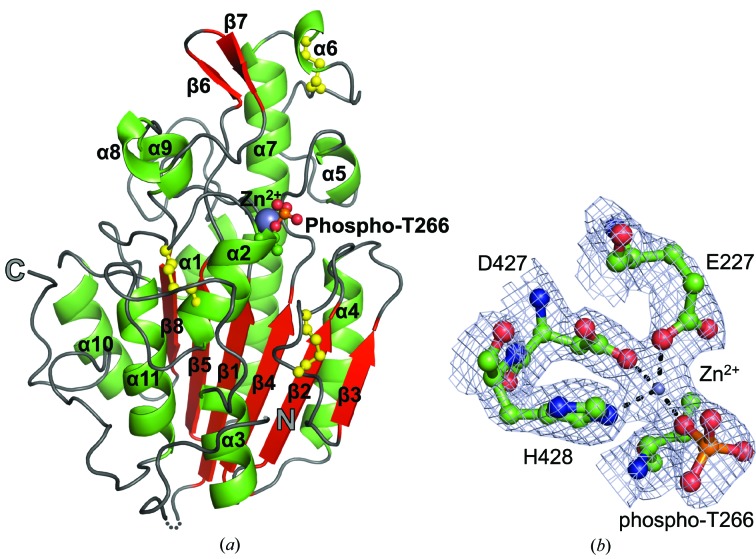
Figure 2.
Structure of the C-terminal catalytic domain of the pEtN transferase EptC (cEptC) from C. jejuni. (a) Secondary-structural features of a monomer. α-Helices (green), β-strands (red) and loops (gray) are labeled as in Supplementary Fig. S3. Disulfides (yellow) and the phosphothreonine residue are displayed in ball-and-stick representation, Zn2+ (purple) is displayed as a sphere and the N- and C-termini are labeled. The dotted line indicates residues 213–215, for which there is no observable electron density in monomer A. (b) Zinc-binding residues of the phosphoenzyme intermediate. 2F o − F c map density (blue mesh) is contoured at 2.0 r.m.s.d. The metal ion was identified as Zn2+ by anomalous diffraction (see Supplementary Fig. S7). Coordination bonds between side chains and Zn2+ are indicated by dashed lines. All molecular images were generated in PyMOL (Schrödinger).