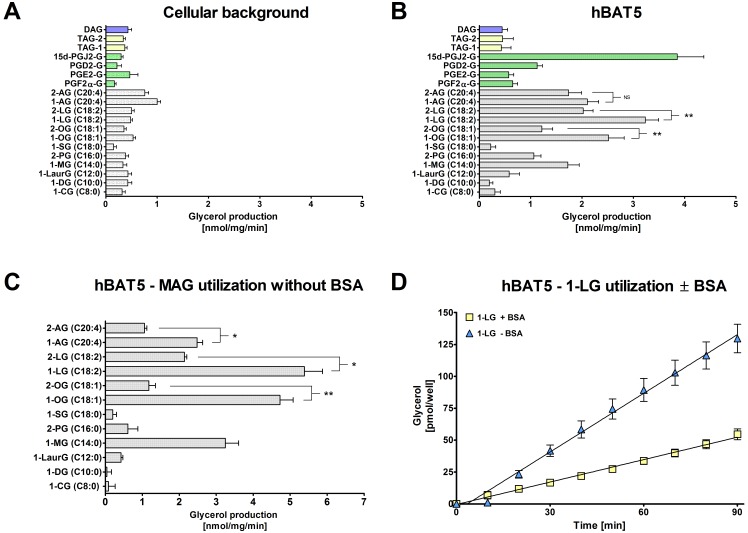
Figure 3. In vitro glycerolipid substrate profile of hBAT5.
HEK293 cells were transiently transfected with the cDNA encoding hBAT5, as detailed in the Methods section. After 48 h, cells were harvested and lysates prepared for hydrolase activity measurements using a sensitive fluorescent glycerol assay. The substrate panel included monoacylglycerols (MAGs) with the indicated acyl chain length, isomer and degree of saturation, the diacylglycerol (DAG) 1,2-dioleoyl(C18∶1)-rac-glycerol, the triacylglycerols (TAG-1 = 1,2,3-trioleoyl(C18∶1)glycerol; TAG-2 = 1-palmitoyl(C16∶0)-2-oleoyl(C18∶1)-3-linoleoyl(C18∶2)-rac-glycerol, as well as the prostaglandin glycerol esters PGD2-G, PGE2-G, PGF2α-G and 15d-PGJ2-G. Cellular lysates (0.3 µg/well) were incubated together with the indicated substrates [25 µM final concentration, added from 10 mM stock solutions in ethanol into the glycerol assay mix containing 0.5% (w/v) fatty acid free BSA and 1% (v/v ethanol). Glycerol production was determined at time-point 60 min. A. Background activity for the tested substrates. Cellular background was similar between HEK and Mock-transfected cells and the values shown are combined from the two. B. Substrate profile of hBAT5. C. The MAG substrate profile of hBAT5 in assays conducted without BSA. D. Linear, time-dependent generation of glycerol as a result of 1-LG (25 µM) hydrolysis in hBAT5-HEK lysates (0.3 µg/well) in incubations with or without BSA. Note ∼2.5-fold higher hydrolysis rate in the absence of BSA. Data are mean + SEM from 3–7 (A and B) or three (C and D) independent experiments using hBAT5 lysates from one transfection. Statistical comparisons between the MAG 1(3)- and 2-isomers were done by using unpaired (B) or paired (C) t-test and the significance is indicated with an asterix (NS, non-significant; *, p<0.05; **, p<0.01).