Abstract
Background
Primary testicular diffuse large B-cell lymphoma (DLBCL) is a rare but aggressive extranodal lymphoma, and its relapse in the central nervous system (CNS) is a major concern during treatment. Despite this, the role of intrathecal prophylaxis in primary testicular DLBCL remains controversial.
Methods
We retrospectively reviewed the medical records of 14 patients with primary testicular DLBCL diagnosed between November 2000 and June 2012, and analyzed the CNS relapse rate in patients treated without intrathecal prophylaxis. Survival curves were estimated using the Kaplan-Meier method.
Results
The median age at diagnosis was 57 years (range, 41-79 years). Unilateral testicular involvement was observed in 13 patients. Nine patients had stage I, 1 had stage II, and 4 had stage IV disease. The international prognostic index was low or low-intermediate risk in 12 patients and high-intermediate risk in 2 patients. Thirteen patients underwent orchiectomy. All the patients received systemic chemotherapy without intrathecal prophylaxis, and prophylactic radiotherapy was administered to the contralateral testis in 12 patients. The median follow-up period of surviving patients was 39 months (range, 10-139 months). Median overall survival was not reached and the median progression-free survival was 3.8 years. Four patients experienced relapse, but CNS relapse was observed in only one patient (7.1%) with stage IV disease, 27 months after a complete response.
Conclusion
Even without intrathecal prophylaxis, the rate of relapse in the CNS was lower in the Korean patients with primary testicular DLBCL compared to prior reports.
Keywords: Diffuse large B cell lymphoma, Intrathecal prophylaxis, Primary testicular lymphoma
INTRODUCTION
Primary testicular lymphoma (PTL) is a rare extranodal lymphoma, accounting for 1% to 2% of all non-Hodgkin lymphomas (NHL) and 4% of all extranodal NHLs [1, 2, 3]. About 65%-90% of PTL patients have diffuse large B-cell lymphoma (DLBCL) [1, 4, 5, 6]. Although the prognosis of DLBCL has improved with the advent of rituximab, PTL remains a hard-to-treat disease with a relatively poor prognosis. Relapse in the central nervous system (CNS) is an issue of particular concern in the treatment of PTL. However, the role of intrathecal chemotherapy in preventing CNS relapse remains controversial [7, 8]. Recently, a study from the International Extranodal Lymphoma Study Group (IELSG) reported that R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisolone) chemoimmunotherapy with intrathecal methotrexate prophylaxis and radiotherapy to the contralateral testis could reduce CNS relapse (6%) compared with a historical control [9, 10]. However, it is difficult to distinguish between the treatment effects of intrathecal methotrexate and rituximab and/or radiotherapy to the contralateral testis. The CNS relapse rate in PTL patients who had undergone rituximab+chemotherapy without intrathecal prophylaxis during the rituximab era has not yet been reported. To address the need for CNS prophylaxis in these patients, we evaluated the CNS relapse rate in PTL patients who underwent CHOP±rituximab chemotherapy without intrathecal prophylaxis.
MATERIALS AND METHODS
Patients
Patients were identified by searching the Asan Medical Center NHL registry databases for PTL. Between November 2000 and June 2012, 14 patients were newly diagnosed as having DLBCL as a form of PTL. We retrospectively reviewed the computerized medical records of these patients. The institutional review board of Asan Medical Center approved this study (IRB No. 2013-0528).
Diagnosis
Diagnosis was based on the morphological and immunohistochemical examination of testicular biopsy samples. Histopathological classification was based on the World Health Organization (WHO) criteria for NHL [11]. PTL was diagnosed, if the testicles were the primary site of the disease or at least the main site of involvement, with either no or only minor nodal involvement [12]. The patients had no prior therapy for lymphoma before diagnosis.
Staging
Staging evaluation for each patient included physical examination; computed tomography (CT) scans of the neck, thorax, abdomen, and pelvis; bilateral bone marrow aspiration and biopsy; lumbar puncture; and positron emission tomography-computed tomography (PET-CT). Patients were staged according to the Ann Arbor staging classification. The international prognostic index (IPI) was assessed as previously described [13].
Response evaluation and follow-up
We assessed therapeutic response using the Revised Response Criteria for Lymphoma [14]. History taking, physical examination, and routine follow-up contrast enhanced neck, chest, and abdominopelvic CT, and PET-CT were performed every 3 months for the first 2 years, every 6 months for the next 3 years, and yearly thereafter or whenever clinically indicated after achieving a complete response (CR).
Statistical analysis
Overall survival (OS) was calculated from the date of diagnosis until the time of death as a result of any cause, or the date of the last follow-up for surviving patients. Progression-free survival (PFS) was calculated from the date of diagnosis to progression of disease or death as a result of any cause. If patients had not progressed or died, PFS was censored at the time of last follow-up. Both OS and PFS curves were plotted using the Kaplan-Meier method. The log-rank test was used to compare survival rates between groups of patients divided according to clinical characteristics. Statistical analyses were performed using the Statistical Package for Social Sciences version 20.0 (SPSS, Chicago, IL).
RESULTS
Patient characteristics
All of the patients initially presented with painless testicular swelling. The median age at diagnosis was 57 years (range, 41-79 years). The left testicle was involved in 2 patients (14.3%), the right testicle in 11 patients (78.6%), and both testicles in 1 patient (7.1%). All patients had an Eastern Cooperative Oncology Group Performance Status (ECOG PS) score of 1. Nine patients (64.3%) had stage I, 1 patient (7.1%) had stage II, and the other 4 patients (28.6%) had stage IV disease. The 4 patients with stage IV disease had extranodal involvement of the bone (N=1), bone marrow (N=1), lung (N=1), and Waldeyer's ring + bone + bone marrow + adrenal gland (N=1). The IPI score was 0-1 (low risk group) in 6 patients (42.9%), 2 (low-intermediate risk group) in 6 patients, and 3 (high-intermediate risk group) in 2 patients (14.3%). No patient had B symptoms. The serum lactate dehydrogenase (LDH) level was elevated in 6 patients. Patient characteristics are summarized in Table 1, and individual characteristics are listed in Table 2.
Table 1.
Patient characteristics, treatment modalities, and outcomes.
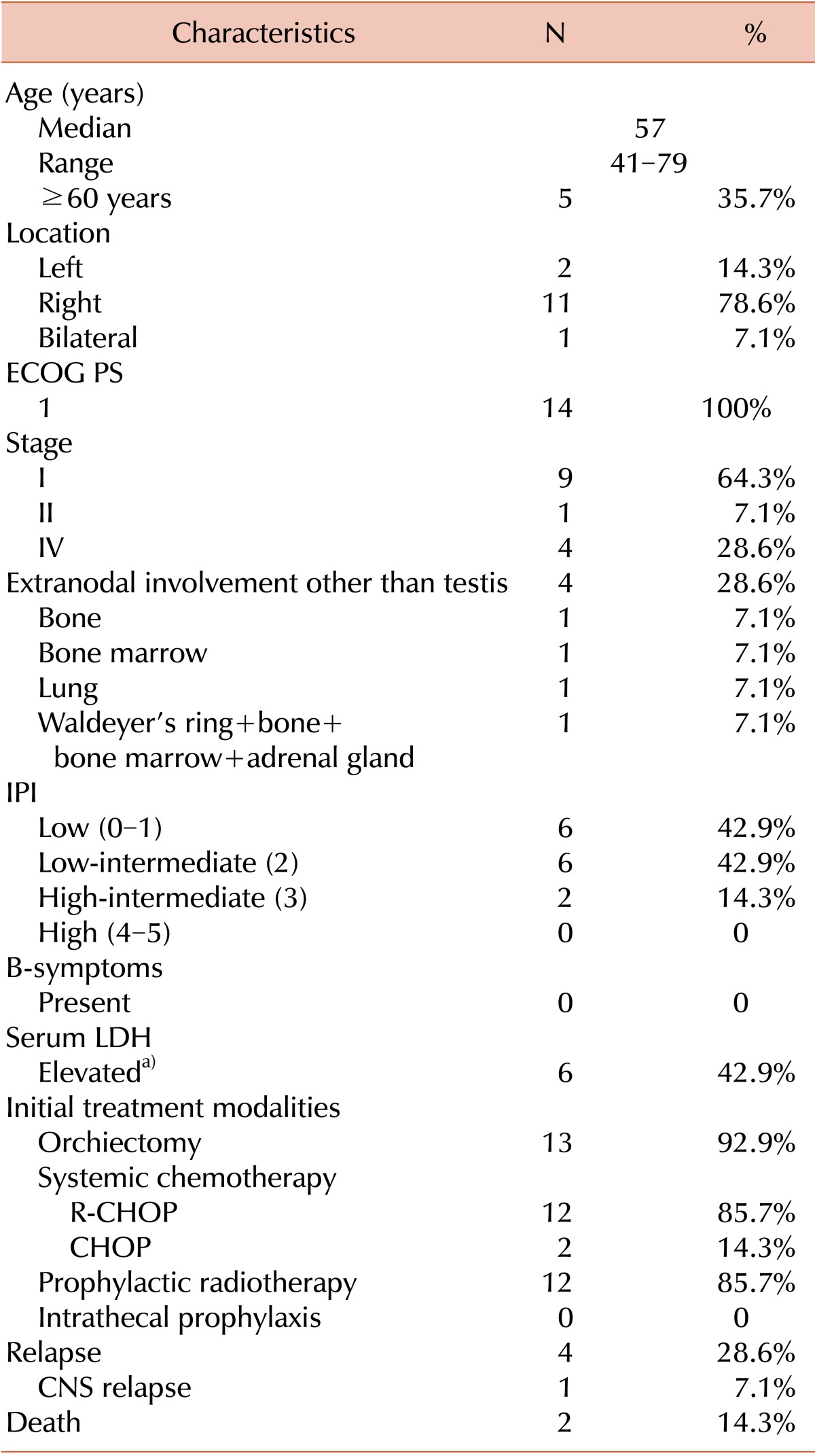
a)Elevated LDH: >250 IU/L.
Abbreviations: ECOG PS, Eastern Cooperative Oncology Group Performance Status; IPI, International Prognostic index; LDH, lactate dehydrogenase; R-CHOP, rituximab+cyclophosphamide+doxorubicin+vincristine+prednisolone; CNS, central nervous system.
Table 2.
Clinical characteristics, treatment modalities, and outcomes for each patient.
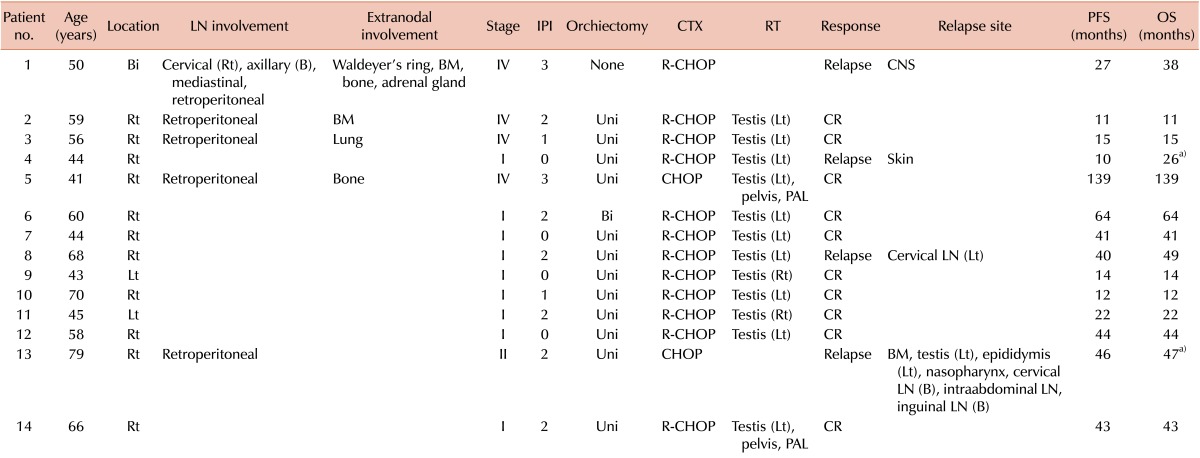
a)Two patients died.
Abbreviations: LN, lymph node; IPI, International Prognostic Index; LDH, lactate dehydrogenase; CTX, chemotherapy; RT, radiotherapy; PFS, progression-free survival; OS, overall survival; Uni, unilateral; Bi, bilateral; Rt, right; Lt, left; B, both; BM, bone marrow; CNS, central nervous system; R-CHOP, rituximab+cyclophosphamide+doxorubicin+vincristine+prednisolone; CR, complete response; PAL, paraaortic lymph node.
Treatment
Thirteen patients (92.9%) underwent orchiectomy as an initial diagnostic and therapeutic procedure. All patients underwent chemotherapy, consisting of either R-CHOP (N=12, 85.7%) or CHOP (N=2, 14.3%) before introduction of rituximab. Prophylactic radiotherapy was administered to the contralateral testis once daily with 5 fractions per week for 12 of the 14 patients with unilateral testicular involvement. The median radiation dose was 3,200 cGy (range, 2,400-3,600 cGy) in 17.6 fractions (range, 12-20 fractions). The remaining 2 patients did not receive prophylactic radiotherapy; one of these patients initially presented with extensive disease (stage IV) and the other was aged 79 years with stage II disease. None of the patients underwent intrathecal prophylaxis, and only one patient with stage IV disease received therapeutic intrathecal methotrexate, after relapse at the leptomeninges was confirmed.
In total, 9 patients with stage I disease and 3 with stage IV disease underwent trimodal treatments comprising orchiectomy, CHOP±R chemotherapy and prophylactic radiotherapy to the contralateral testis. One patient with stage II disease underwent bimodal treatment involving orchiectomy and CHOP chemotherapy, but not prophylactic radiotherapy at the discretion of the treating physician. The remaining patient with stage IV disease received R-CHOP chemotherapy only. Initial treatments are summarized in Table 1 and individual treatment modalities are listed in Table 2.
Responses and outcomes
All patients achieved a CR after completion of first-line chemotherapy. The median duration of follow-up in the surviving patients was 39 months (range, 10-139 months). Lymphoma recurred in 4 patients (28.6%). One patient with stage II disease at diagnosis, who was not administered prophylactic radiotherapy to the contralateral testis, experienced a relapse at multiple sites including the bone marrow, contralateral testis, epididymis, nasopharynx, and both the cervical and intra-abdominal lymph nodes. He died of sepsis after the first cycle of second-line chemotherapy (DHAP, dexamethasone, cytarabine, and cisplatin). Another patient with stage IV disease at diagnosis, who was given R-CHOP chemotherapy only, experienced relapse at the spinal nerve roots and leptomeninges 27 months after initially achieving a CR. He was then administered intrathecal methotrexate, and high-dose methotrexate/cytarabine chemotherapy, although refractory disease persisted. The other 2 patients with stage I disease at diagnosis, who underwent orchiectomy+R-CHOP chemotherapy+prophylactic radiotherapy to the contralateral testis, suffered from relapses, one in the left cervical lymph nodes and the other in the skin of the left upper arm. One patient with relapse at the cervical lymph node achieved a second CR after 6 cycles of ESHAP chemotherapy (etoposide, methylprednisolone, cytarabine, and cisplatin). The other patient underwent excision biopsy for the skin involvement. Although the tumor showed regression after excision, he experienced relapse in the skin of the thigh and stomach 5 months after excision. He showed progressive disease despite the administration of ESHAP chemotherapy and autologous stem cell transplantation, and he died of progressive disease. The treatment responses and outcomes of individual patients are listed in Table 2, and a flowchart summarizing the treatment of all patients is shown in Fig. 1.
Fig. 1.
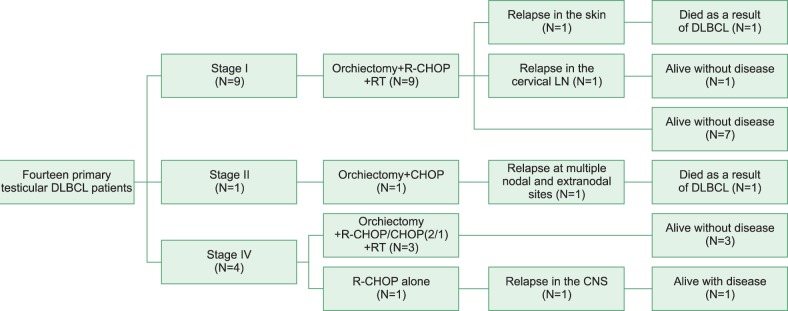
Treatment flowchart of 14 patients with primary testicular DLBCL. Abbreviations: R-CHOP, rituximab+cyclophosphamide+doxorubicin+vincristine+prednisolone; RT, radiotherapy; LN, lymph node; CNS, central nervous system; DLBCL, diffuse large B-cell lymphoma.
The median PFS was 3.8 years, with a 3-year PFS rate of 81%. No median OS had been reached at the time of reporting, but the 3-year OS rate was 89% (Fig. 2). In univariate analysis, none of the patient characteristics-including an older age (≥60 years old), bilateral testicular involvement, left testicular involvement, advanced stage, extranodal involvement other than testis, a high-intermediate or high risk IPI, elevated LDH level, and prophylactic radiotherapy-were found to be significantly associated with PFS and/or OS (Table 3). The ECOG PS was 1 for all patients, and was therefore not analyzed. Multivariate analysis was not performed due to the small sample size and number of events.
Fig. 2.
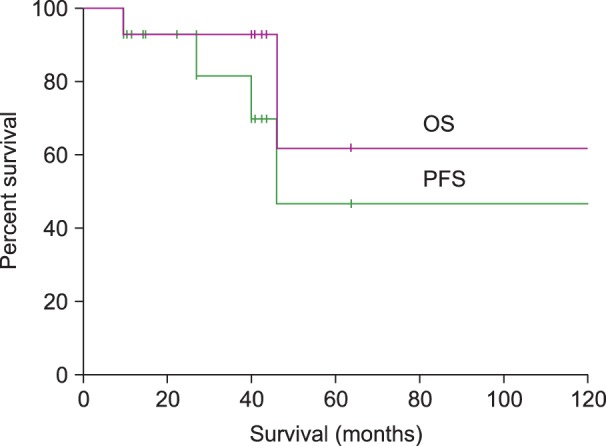
Kaplan-Meier plots for progression-free and overall survival. Abbreviations: OS; overall survival, PFS; progression-free survival.
Table 3.
Univariate analysis of OS and PFS according to clinical characteristics.

Abbreviations: OS, overall survival; PFS, progression-free survival; IPI, International Prognostic index; L, low; LI, low-intermediate; HI, high-intermediate; H, high; LDH, lactate dehydrogenase; RT, radiotherapy.
DISCUSSION
In this retrospective study, all patients with primary testicular DLBCL underwent CHOP ± R without intrathecal prophylaxis and achieved CR after initial treatment. Four patients suffered from a relapse of lymphoma, but this occurred in the CNS in only a single patient (7.1%). This CNS relapse rate is lower than those (9%-35%) previously reported [4, 6, 10, 15]. The patient with CNS relapse initially presented with stage IV disease with multiple nodal and extranodal involvements in Waldeyer's ring, bone marrow, bone, both testicles, and the adrenal gland, and also had an elevated LDH level. The patient showed simultaneous relapse at the spinal nerve roots and leptomeninges 27 months after remission. Notably, none of the patients with stage I or II disease experienced CNS relapse.
Retrospective studies on PTL conducted at the IELSG and M.D. Anderson Cancer Center (MDACC) showed that the rate of CNS relapse was not significantly affected by the administration of intrathecal prophylaxis [10, 15]. The IELSG-10 study, which was the first prospective study on PTL, showed that multimodal therapy with R-CHOP, intrathecal methotrexate, and testicular radiotherapy can produce a good survival outcome and low CNS relapse rate (6%). Nonetheless, the IELSG-10 study could not distinguish between the effects of intrathecal prophylaxis and combined rituximab with radiotherapy to the contralateral testis on the prevention of CNS relapse [9]. The CNS relapse rate observed in the present study was similar to that in the IELSG-10 study even without intrathecal prophylaxis, and is lower than those reported from previous retrospective studies [4, 6, 9, 10, 15].
A potential sanctuary site formed by the blood-testis barrier makes testicular tumors inaccessible to systemic chemotherapy, but can be removed by orchiectomy. However, considering that most patients suffer from relapse within 2 years, orchiectomy alone is not a definite treatment, even in patients with stage I disease [16]. The IELSG study reported that the continuous risk of contralateral testicular relapse was 15% at 3 years and 42% at 15 years in patients treated without prophylactic testicular irradiation. Furthermore, prophylactic testicular irradiation could reduce the rate of testicular relapse, from 35% to 8% [10]. In addition to systemic chemotherapy, orchiectomy and prophylactic testicular irradiation were associated with an improved PFS and OS rate, however, its specific effect on CNS relapse was not statistically significant [10, 15].
The low CNS relapse rate in our study might be explained at least in part by the inclusion of rituximab (85.7% of patients) or the application of multimodal treatment (85.7% of patients with orchiectomy+chemotherapy+radiotherapy). The role of systemic rituximab in the prevention of CNS relapse is somewhat controversial [13, 17, 18, 19, 20, 21]. A randomized controlled trial on elderly patients with aggressive B-cell lymphoma (RICOVER-60) demonstrated that the addition of rituximab could significantly reduce the CNS relapse rate (4% vs. 6%, P=0.043) [17]. Given that the cerebrospinal fluid (CSF) rituximab concentration after intravenous administration was less than 1% of its plasma level, improved systemic disease control by rituximab might have contributed to the prevention of CNS relapse [22].
The rate of CNS progression has been reported to be up to 30% amongst patients with advanced stage disease, and about 10% amongst those with limited stage disease [23, 24]. It has therefore been suggested that CNS prophylaxis is warranted in advanced disease and may be considered in limited disease [3]. However, there is no prospective study, which clarifies the role of intrathecal prophylaxis according to the extent of disease. In our study, CNS relapse occurred in a patient who had disseminated disease at presentation, suggesting that micrometastasis could have been present when PTL was diagnosed, or the disease could have progressed after treatment.
Intrathecal chemotherapy has several limitations [7]. First, given that the brain parenchyma is a more frequent site of CNS involvement than the leptomeninges in PTL, prophylactic intrathecal chemotherapy might be ineffective [4, 10]. Furthermore, the tissue concentration of chemotherapeutic agents rapidly decreases as distance from the ependymal surface increases [25]. Second, intrathecal chemotherapy using lumbar puncture can result in a variable ventricular methotrexate concentration, and there may be occasional epidural or subdural leakage [26]. The frequent use of Ommaya reservoirs for drug delivery might cause fatal complications, such as hemorrhage. Finally, chemotherapeutic agents are rapidly eliminated from CSF by flow excretion and detoxified in the choroid plexus [27].
Recently, the introduction of rituximab and the use of multimodal treatment have improved the systemic disease control of primary testicular DLBCL, although the role of intrathecal prophylaxis in preventing CNS relapse amongst these patients remains controversial [7, 8]. However, based on previous reports, intrathecal chemotherapy is routinely performed at many institutions, although many of these studies were conducted during the pre-rituximab era [1, 10, 15]. The study we report here is the first to address the role of intrathecal prophylaxis in the rituximab era. We found that the rate of CNS relapse is no higher than that in previous reports, even without intrathecal prophylaxis, and we think that a prospective study with a larger population of patients is warranted to conclusively demonstrate our findings.
This retrospective study has some limitations. The small study population, diverse treatment protocols, and relatively short follow-up period made it difficult to identify relevant prognostic factors. Poor prognostic markers reported in previous studies include older age, advanced-stage disease, high ECOG PS scores, infiltration of adjacent tissues, bulky disease, elevated LDH, the presence of B symptoms, a high IPI score, left testicular involvement, and not having surgery or radiotherapy [1, 4, 28, 29, 30]. However, we could not demonstrate the association of those clinical characteristics with clinical outcomes in this study due to the small sample size.
In conclusion, even without intrathecal prophylaxis, the rate of CNS relapse is relatively low in the Korean patients with primary testicular DLBCL, compared to previous reports.
Footnotes
No potential conflicts of interest relevant to this article were reported.
References
- 1.Moller MB, d'Amore F, Christensen BE The Danish Lymphoma Study Group, LYFO. Testicular lymphoma: a population-based study of incidence, clinicopathological correlations and prognosis. Eur J Cancer. 1994;30A:1760–1764. doi: 10.1016/0959-8049(94)00311-r. [DOI] [PubMed] [Google Scholar]
- 2.Shahab N, Doll DC. Testicular lymphoma. Semin Oncol. 1999;26:259–269. [PubMed] [Google Scholar]
- 3.Zucca E, Roggero E, Bertoni F, Cavalli F. Primary extranodal non-Hodgkin's lymphomas. Part 1: Gastrointestinal, cutaneous and genitourinary lymphomas. Ann Oncol. 1997;8:727–737. doi: 10.1023/a:1008282818705. [DOI] [PubMed] [Google Scholar]
- 4.Park BB, Kim JG, Sohn SK, et al. Consideration of aggressive therapeutic strategies for primary testicular lymphoma. Am J Hematol. 2007;82:840–845. doi: 10.1002/ajh.20973. [DOI] [PubMed] [Google Scholar]
- 5.Sussman EB, Hajdu SI, Lieberman PH, Whitmore WF. Malignant lymphoma of the testis: a clinicopathologic study of 37 cases. J Urol. 1977;118:1004–1007. doi: 10.1016/s0022-5347(17)58277-4. [DOI] [PubMed] [Google Scholar]
- 6.Lantz AG, Power N, Hutton B, Gupta R. Malignant lymphoma of the testis: a study of 12 cases. Can Urol Assoc J. 2009;3:393–398. doi: 10.5489/cuaj.1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kridel R, Dietrich PY. Prevention of CNS relapse in diffuse large B-cell lymphoma. Lancet Oncol. 2011;12:1258–1266. doi: 10.1016/S1470-2045(11)70140-1. [DOI] [PubMed] [Google Scholar]
- 8.Tomita N, Kodama F, Kanamori H, Motomura S, Ishigatsubo Y. Prophylactic intrathecal methotrexate and hydrocortisone reduces central nervous system recurrence and improves survival in aggressive non-hodgkin lymphoma. Cancer. 2002;95:576–580. doi: 10.1002/cncr.10699. [DOI] [PubMed] [Google Scholar]
- 9.Vitolo U, Chiappella A, Ferreri AJ, et al. First-line treatment for primary testicular diffuse large B-cell lymphoma with rituximab-CHOP, CNS prophylaxis, and contralateral testis irradiation: final results of an international phase II trial. J Clin Oncol. 2011;29:2766–2772. doi: 10.1200/JCO.2010.31.4187. [DOI] [PubMed] [Google Scholar]
- 10.Zucca E, Conconi A, Mughal TI, et al. Patterns of outcome and prognostic factors in primary large-cell lymphoma of the testis in a survey by the International Extranodal Lymphoma Study Group. J Clin Oncol. 2003;21:20–27. doi: 10.1200/JCO.2003.11.141. [DOI] [PubMed] [Google Scholar]
- 11.Swerdlow SH, Campo E, Harris NL, et al., editors. WHO classification of tumours of haematopoietic and lymphoid tissue. 4th ed. Lyon, France: IARC Press; 2008. [Google Scholar]
- 12.Krol AD, le Cessie S, Snijder S, Kluin-Nelemans JC, Kluin PM, Noordijk EM. Primary extranodal non-Hodgkin's lymphoma (NHL): the impact of alternative definitions tested in the Comprehensive Cancer Centre West population-based NHL registry. Ann Oncol. 2003;14:131–139. doi: 10.1093/annonc/mdg004. [DOI] [PubMed] [Google Scholar]
- 13.The International Non-Hodgkin's Lymphoma Prognostic Factors Project. A predictive model for aggressive non-Hodgkin's lymphoma. N Engl J Med. 1993;329:987–994. doi: 10.1056/NEJM199309303291402. [DOI] [PubMed] [Google Scholar]
- 14.Cheson BD, Pfistner B, Juweid ME, et al. Revised response criteria for malignant lymphoma. J Clin Oncol. 2007;25:579–586. doi: 10.1200/JCO.2006.09.2403. [DOI] [PubMed] [Google Scholar]
- 15.Mazloom A, Fowler N, Medeiros LJ, Iyengar P, Horace P, Dabaja BS. Outcome of patients with diffuse large B-cell lymphoma of the testis by era of treatment: the M. D. Anderson Cancer Center experience. Leuk Lymphoma. 2010;51:1217–1224. doi: 10.3109/10428191003793358. [DOI] [PubMed] [Google Scholar]
- 16.Vitolo U, Ferreri AJ, Zucca E. Primary testicular lymphoma. Crit Rev Oncol Hematol. 2008;65:183–189. doi: 10.1016/j.critrevonc.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 17.Boehme V, Schmitz N, Zeynalova S, Loeffler M, Pfreundschuh M. CNS events in elderly patients with aggressive lymphoma treated with modern chemotherapy (CHOP-14) with or without rituximab: an analysis of patients treated in the RICOVER-60 trial of the German High-Grade Non-Hodgkin Lymphoma Study Group (DSHNHL) Blood. 2009;113:3896–3902. doi: 10.1182/blood-2008-10-182253. [DOI] [PubMed] [Google Scholar]
- 18.Shimazu Y, Notohara K, Ueda Y. Diffuse large B-cell lymphoma with central nervous system relapse: prognosis and risk factors according to retrospective analysis from a single-center experience. Int J Hematol. 2009;89:577–583. doi: 10.1007/s12185-009-0289-2. [DOI] [PubMed] [Google Scholar]
- 19.Villa D, Connors JM, Shenkier TN, Gascoyne RD, Sehn LH, Savage KJ. Incidence and risk factors for central nervous system relapse in patients with diffuse large B-cell lymphoma: the impact of the addition of rituximab to CHOP chemotherapy. Ann Oncol. 2010;21:1046–1052. doi: 10.1093/annonc/mdp432. [DOI] [PubMed] [Google Scholar]
- 20.Tai WM, Chung J, Tang PL, et al. Central nervous system (CNS) relapse in diffuse large B cell lymphoma (DLBCL): pre- and post-rituximab. Ann Hematol. 2011;90:809–818. doi: 10.1007/s00277-010-1150-7. [DOI] [PubMed] [Google Scholar]
- 21.Yamamoto W, Tomita N, Watanabe R, et al. Central nervous system involvement in diffuse large B-cell lymphoma. Eur J Haematol. 2010;85:6–10. doi: 10.1111/j.1600-0609.2010.01438.x. [DOI] [PubMed] [Google Scholar]
- 22.Seymour JF, Solomon B, Wolf MM, Janusczewicz EH, Wirth A, Prince HM. Primary large-cell non-Hodgkin's lymphoma of the testis: a retrospective analysis of patterns of failure and prognostic factors. Clin Lymphoma. 2001;2:109–115. doi: 10.3816/clm.2001.n.016. [DOI] [PubMed] [Google Scholar]
- 23.Turner RR, Colby TV, MacKintosh FR. Testicular lymphomas: a clinicopathologic study of 35 cases. Cancer. 1981;48:2095–2102. doi: 10.1002/1097-0142(19811101)48:9<2095::aid-cncr2820480930>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 24.Touroutoglou N, Dimopoulos MA, Younes A, et al. Testicular lymphoma: late relapses and poor outcome despite doxorubicin-based therapy. J Clin Oncol. 1995;13:1361–1367. doi: 10.1200/JCO.1995.13.6.1361. [DOI] [PubMed] [Google Scholar]
- 25.Blasberg RG, Patlak C, Fenstermacher JD. Intrathecal chemotherapy: brain tissue profiles after ventriculocisternal perfusion. J Pharmacol Exp Ther. 1975;195:73–83. [PubMed] [Google Scholar]
- 26.Shapiro WR, Young DF, Mehta BM. Methotrexate: distribution in cerebrospinal fluid after intravenous, ventricular and lumbar injections. N Engl J Med. 1975;293:161–166. doi: 10.1056/NEJM197507242930402. [DOI] [PubMed] [Google Scholar]
- 27.Fleischhack G, Jaehde U, Bode U. Pharmacokinetics following intraventricular administration of chemotherapy in patients with neoplastic meningitis. Clin Pharmacokinet. 2005;44:1–31. doi: 10.2165/00003088-200544010-00001. [DOI] [PubMed] [Google Scholar]
- 28.Gundrum JD, Mathiason MA, Moore DB, Go RS. Primary testicular diffuse large B-cell lymphoma: a population-based study on the incidence, natural history, and survival comparison with primary nodal counterpart before and after the introduction of rituximab. J Clin Oncol. 2009;27:5227–5232. doi: 10.1200/JCO.2009.22.5896. [DOI] [PubMed] [Google Scholar]
- 29.Booman M, Douwes J, Glas AM, de Jong D, Schuuring E, Kluin PM. Primary testicular diffuse large B-cell lymphomas have activated B-cell-like subtype characteristics. J Pathol. 2006;210:163–171. doi: 10.1002/path.2033. [DOI] [PubMed] [Google Scholar]
- 30.Wang Y, Li ZM, Huang JJ, et al. Three prognostic factors influence clinical outcomes of primary testicular lymphoma. Tumour Biol. 2013;34:55–63. doi: 10.1007/s13277-012-0510-4. [DOI] [PubMed] [Google Scholar]


