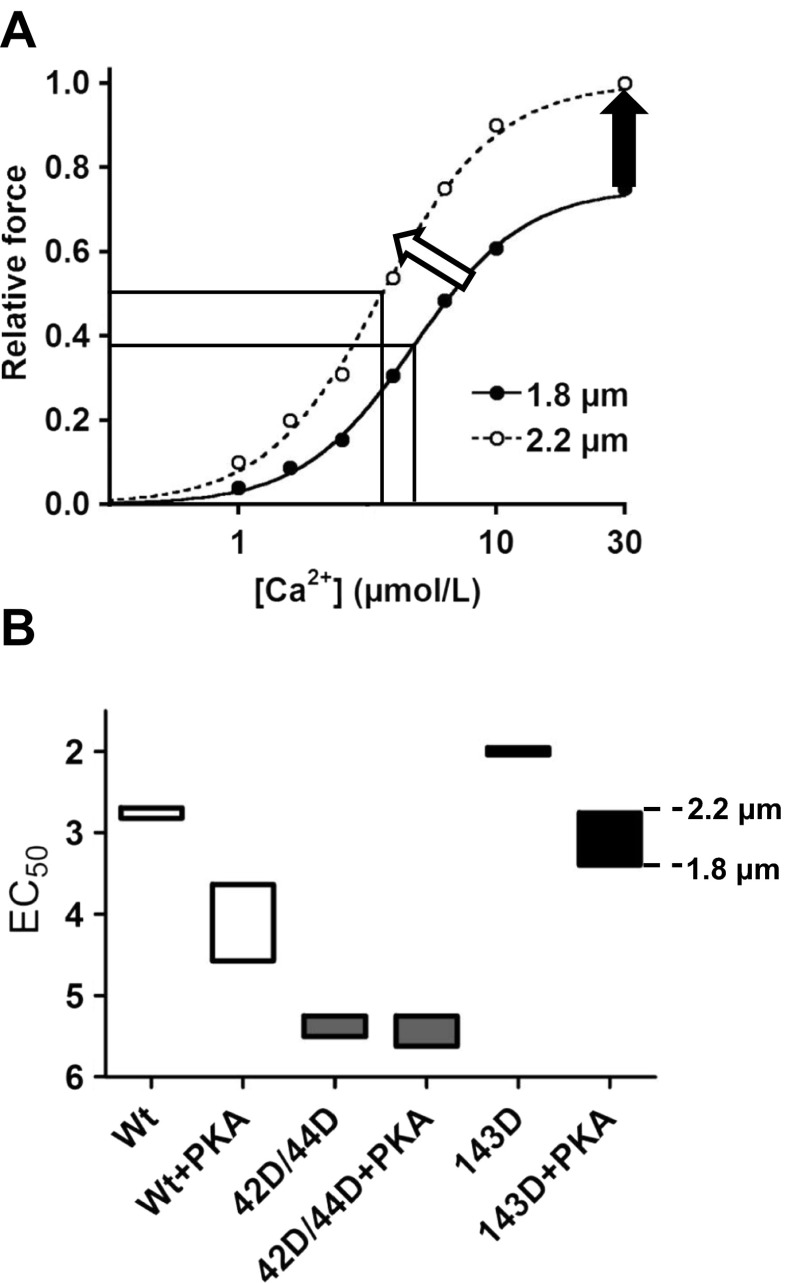
Fig. 2.
Schematic representation of changes in length-dependent Ca2+-sensitivity upon phosphorylation of Ser42/44, Thr143 and/or Ser23/24. a An example of myofilament force development at short (1.8 μm) and long (2.2 μm) sarcomere length at various [Ca2+] in human membrane-permeabilised cardiomyocytes. Cardiomyocyte lengthening from 1.8 μm to 2.2 μm increases myofilament Ca2+ sensitivity (white arrow). The Ca2+ sensitivity derived from the midpoint of the force–Ca2+ relationship (EC50) is demonstrated for both sarcomere lengths by a vertical line, and the difference represents the delta EC50. An increase in left ventricular filling increases myofilament Ca2+ sensitivity and underlies, together with an increase in maximal force-generating capacity (black arrow), increased cardiac output during the subsequent systolic phase. b Data obtained in troponin-exchanged donor cells without and with treatment with exogenous PKA [33, 41] were combined to illustrate the range at which myofilament Ca2+ sensitivity (EC50) may vary in response to phosphorylation at Ser23/24 and the PKC sites Ser42/44 and Thr143. Abbreviations: wild-type (Wt); phosphorylated 42/44 (42D/44D); phosphorylated 143 (143D). Boxes represent the range of Ca2+ sensitivity measured at a sarcomere length of 1.8 (lower line) and 2.2 μm (upper line). This figure demonstrates that the sarcomere length-dependent shift in Ca2+ sensitivity is relatively small in Wt, 42D/44D and 143D without PKA (i.e. low cTnI-Ser23/24 phosphorylation). PKA treatment of Wt and 143D increased the range in Ca2+ sensitivity at which the sarcomere is operating upon changes in sarcomere length between 1.8 and 2.2 μm. However, PKA treatment of 42D/44D does not enhance the length-dependent increase in Ca2+-sensitivity