Table 1.
Thiocarbonylation reaction of 2,4,6-tribenzyl myo-inositol
| Entry | Thionoformate (equiv) | Base (equiv) | Catalyst (20 mol%) | Conversion (%)a |
|---|---|---|---|---|
| 1 |

|
Pyridine (1.2) | - | 20 |
| 2 | Pyridine (1.2) | DMAP | 25 | |
| 3 | 2,6-Lutidine (1.2) | DMAP | <5 | |
|
| ||||
| 4 |
|
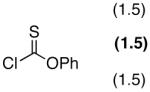
PEMP (2) | - | N.R. |
| 5 | PEMP (2) | NMI | 90 | |
| 6 | PEMP (2) | DMAP | 76 | |
|
| ||||
| 7 |
|
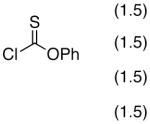
K2CO3 (2) | NMI | N.R. |
| 8 | Proton Sponge (2) | NMI | 13 | |
| 9 | DABCO (2) | NMI | N.R. | |
| 10 | DIPEA (2) | NMI | 45 | |
|
| ||||
| 11 | ClC(S)O-p-tolyl (1.5) | PEMP (2) | NMI | 91 |
| 12 | ClC(S)O-p-C6H4Cl (1.5) | PEMP (2) | NMI | 78 |
| 13 | ClC(S)O-p-C6H4F (1.5) | PEMP (2) | NMI | 76 |
Conversions determined by 1H-NMR integration
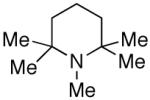
PEMP = 1,2,2,6,6-pentamethyl piperidine: