Abstract
One of the major components of some geraniums is geraniin, described by its discoverer as crystallizable tannin, well known as an excellent antioxidant, and also found in fruits such as pomegranate. Recently, natural antioxidants have attracted great attention from consumers over the world due to their lower toxicity than synthetics. But geraniin is not a stable compound, and also is difficult to obtain, that is why in the present study we obtained acetonylgeraniin from Geranium schideanum (Gs), a stable acetone condensate of geraniin. In the present study the effect of Gs acetone-water extract was studied in reference to postnecrotic liver regeneration induced by thioacetamide (TA) in rats. Two months male rats were pretreated with daily dose of Gs extract for 4 days (300 mg/kg) and the last day also were intraperitoneally injected with TA (6.6 mmol/kg). Samples of blood were obtained from rats at 0, 24, 48, 72 and 96 h following TA intoxication. The pre-treatment with the crude extract in the model of thioacetamide-induced hepatotoxicity in rats decreased and delayed liver injury by 66% at 24 h. This result suggests that Gs extract may be used as an alternative for reduction of liver damage. On the other hand, acute toxicity study revealed that the LD50 value of the Gs extract is more than the dose 5000 mg/kg in rats, according to the Lorke method.
Keywords: Geraniin, Geranium schideanum, thioacetamide hepatotoxicity
INTRODUCTION
Geranium genus is taxonomically classified within the family Geraniaceae Juss, which includes 5 to 11 geniuses, and in total near to 750 species. The best-known geniuses are: Genus Geranium as wild plants and genus Pelargonium as ornamental plants in this family. The traditional use describes their therapeutic properties as an antiseptic in wounds, and antipyretic using the infusion of the plant. Currently, eight different species of geraniums, belongs to the family Geraniaceae, have been identified in Hidalgo State, in Central Mexico,[1] and none of these eight species has chemical or pharmacological studies.
All phytochemical studies described for these species indicate the presence of polyphenolic compounds, especially tannins, which have been considered as water-soluble compounds of molecular weight between 500 and 30,000 g/mol, with special properties such as the ability to precipitate alkaloids, gelatin and other proteins.[2] Nowadays tannins are well known because of their antioxidant properties. Tannin-protein complexes in the gastrointestinal tract provide persistent antioxidant activity.
One of the major components in these plants is geraniin, well known as an excellent antioxidant. Geraniin, a compound that has also been found in fruits such as pomegranate, was described by its discoverer as crystallizable tannin. This substance was isolated from Geranium thunbergi in 1975,[3] has been evaluated and showed biologically antihypertensive activity, inhibits angiotensin-converting enzyme and the reverse transcriptase of RNA tumor viruses; also immunomodulatory activity has been found in it. But geraniin is not a stable compound, and also is difficult to obtain, that is why, in the present study we obtained acetonylgeraniin from Geranium schideanum (Gs), a stable acetone condensate of geraniin easily obtainable from the acetone-water extract. On the other hand, Kaempferol, another compound found in geraniums, has been described as a flavonoid, a natural antioxidant. Those and other compounds have been found in some Geranium species in our group, like in Geranium bellum[4,5] or in the present study in Gs, probed the significant role that this species could have in the prevention and treatment of chronic degenerative diseases like liver cirrhosis, hypertension, diabetes, cancer, obesity, kidney diseases or neurodegenerative diseases. Moreover, one example of the hepatoprotection power of geranium genus was found in Geranium macrorrhizum, the methanol extracts, administered were evaluated in a CCl4-induced hepatotoxicity model. The administered methanol extracts with the highest antioxidant potential showed a significant dose-dependent hepatoprotective action against CCl4-induced liver damage decreasing the levels of liver transaminases.[6]
Geranium schiedeanum (Gs), is a perennial plant with long roots, found in the grassy meadows bordering pine/oak forests in the mountains of Hidalgo State, Mexico, where it has the popular name “pata de leσn” and has been used as a traditional remedy for treatment of fevers, pain, and gastrointestinal disorders. The use of other Geranium species has also been reported with hypoglycemic, antihypertensive and cholesterol-lowering effect. However, scientific evidence does not exist in any literature to corroborate these targets or any other. In the present study the effects of Gs were studied in reference to postnecrotic liver damage induced by thioacetamide (TA). Thioacetamide (TA) is a potent hepatotoxic agent which, when administered at doses of 500 mg/kg to rats, initiates a severe hepatocellular perivenous necrosis.[7] The selective destruction of perivenous hepatocytes and the proliferative state of liver cells that immediately follows have been used as an experimental model to study the hepatic response against the aggressive attack of a hepatotoxic drug.
EXPERIMENTAL SECTION
Plant material
Specimen of Geranium schiedeanum was collected at Epazoyucan Municipality, in Hidalgo State, México, during June 2011; it was identified by Professor Manuel González Ledesma. A voucher specimen (J. A. Gayosoo-de-Lucio 06) is preserved at the Herbarium of Biological Research Centre, Autonomous University of Hidalgo, Pachuca, Hidalgo, Mexico.
Extraction and purification
Air-dried aerial parts (1 kg) were extracted with acetone-H2O 7:3 (20 L) by maceration for 7 days. Vacuum evaporation of dissolvent gave 5 L of aqueous residue, filtration of this residue gave a fatty solid residue (12 g) and complete evaporation of water gave the acetone-water extract (115 g). Three grams of acetone-water extract were purified on a Sephadex LH-20 (25 g) column (2.5 * 60 cm) using H2O, H2O-MeOH (9:1, 4:1, 7:3, 3:2, 1:1, 2:3, 3:7, 1:4, 1:9) and MeOH, as eluents. Fractions of 300 mL of each polarity were collected and marked “A-K”. They were evaporated and analyzed by TLC (CHCl3-MeOH-H2O, 5:1.8:0.4) and NMR. Fractions “B” gave 75 mg, and were purified over silica gel (10 g), column (2 * 60 cm) using CHCl3, CHCl3-AcOEt (9:1, 4:1, 7:3, 3:2, 1:1, 2:3, 3:7, 1:4, 1:9) and AcOEt (10 mL of each polarity), as eluents and collecting fractions of 7 mL, fractions 13-16 gave I 25 mg. Fractions “C” and “D” gave 56 mg and were purified over silica gel (10 g) column (2 * 60 cm) using CHCl3-MeOH (50:7.0, 48:7, 45:7, 40:7, 35:7 and 30:7, 40 mL of each) as eluents and collecting fractions of 7 mL, fractions 33-66 gave compound II, 2 mg ( = −168° c = 0.0098 in MeOH), these procedure was repeated ten times to obtain 18 mg of compound II. Fractions “F-I” gave 1.8 g, a portion of this fraction (500 mg) was purified over silica gel C-18 (5 g) column (1 * 60 cm) using H2O, H2O-MeOH (9:1, 4:1, 7:3, 3:2, 1:1, 2:3, 3:7, 1:4, 1:9) and MeOH (20 mL of each polarity), fractions of 10 mL were collected. Fractions 2 to 4 gave 330 mg of compound III. Fraction “K” gave 90 mg, to this fraction we added 5 mL of hot pyridine (40°) and were placed at room temperature for 72 h, filtrated of this mixture give a yellow needles (60 mg) of compound IV [Figure 1].
= −168° c = 0.0098 in MeOH), these procedure was repeated ten times to obtain 18 mg of compound II. Fractions “F-I” gave 1.8 g, a portion of this fraction (500 mg) was purified over silica gel C-18 (5 g) column (1 * 60 cm) using H2O, H2O-MeOH (9:1, 4:1, 7:3, 3:2, 1:1, 2:3, 3:7, 1:4, 1:9) and MeOH (20 mL of each polarity), fractions of 10 mL were collected. Fractions 2 to 4 gave 330 mg of compound III. Fraction “K” gave 90 mg, to this fraction we added 5 mL of hot pyridine (40°) and were placed at room temperature for 72 h, filtrated of this mixture give a yellow needles (60 mg) of compound IV [Figure 1].
Figure 1.
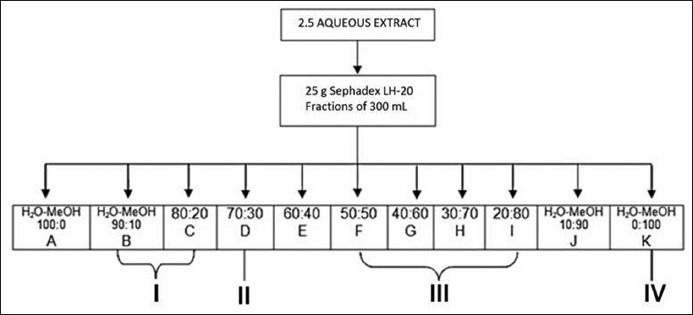
Extract fractions scheme.
Animals
Experiments were performed on male adult Wistar rats 2 months old (200-220g), obtained from Bioterio-UAEH. All experiments were approved by an internal Ethical Institutional Committee for the Care and Use of Laboratory Animals (Comité Institucional Éticopara el Cuidado y Uso de Animales de Laboratorio) and were carried out following the Mexican Official Norm for Animal Care and Handing (NOM-062-ZOO-1999). The animals were housed in a climate and light controlled room with a 12 h light/dark cycle. Rats were allowed free access to food (standard pellet diet) and water ad libitum. The number of experimental animals was kept to a minimum, and at the end of the study, the animals were sacrificed in a CO2 chamber.
Thioacetamide-induced hepatotoxicity study
Male adult Wistar rats 2 months old (200-220g) were acclimated in an animal room for 2 weeks before use. Throughout these 2 weeks rats were supplied with food and water ad libitum. Wistar rats were intragastric pre-treated or not with a single dose of Gs extract (300 mg/kg) during 4 days, at the fourth day of pretreatment also were intraperitoneally injected with a single dose of TA (6.6 mmol/kg) freshly dissolved in 0.9% NaCl. The dose of thioacetamide was chosen as the highest dose with survival above 90%.[8,9] Samples of blood and liver were obtained from rats at 0, 24, 48, 72 and 96 h following TA intoxication. Untreated animals received 0.5 ml of 0.9% NaCl. Experiments were performed on two different groups: Rats treated with a single dose of thioacetamide (TA) and rats pre-treated with Gs and treated with a single dose of thioacetamide (Gs + TA). Each experiment was performed in duplicate from four different animals and followed the international criteria for the use and care of experimental animals outlined in The Guiding Principles in the use of Animals in Toxicology adopted by the Society of Toxicology in 1989. The administered dose showed a significant dose-dependent hepatoprotective action.
Acute toxicity test
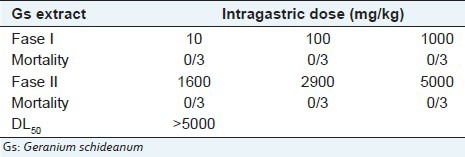
The method described by Lorke (1983) was employed in the determination of the median lethal dose (LD50) of the Gs extract in rats. The extract was suspended in vehicle (distilled water) and the concentrations were adjusted to orally administrate 0.5 mL/100 g body weight. The method was divided into two phases; in the first phase, rats were weighed and randomly divided into four groups each containing three rats. Three groups received Gs extract at doses of 10, 100 and 1000 mg/kg, respectively. The control group was treated only with distilled water orally administered. Based on the result of the first phase, on the second phase three groups of one rat per group were weighed and administered with doses of 1600, 2900 and 5000 mg/kg, respectively. In both phases, rats were weighed and observed daily during 14 days for mortality, any abnormal behavior and other toxic signs. All surviving animal were sacrificed at the end of each test and then autopsied and examined macroscopically for any pathological changes compared with the control group.
Processing of samples
In order to clarify the sequential changes during the different stages of liver injury and the post-necrotic regenerative response, samples were obtained from control and at 0, 24, 48, 72 and 96 h following TA intoxication in both Gs pre-treated or non-pre-treated animals. Rats were sacrificed by cervical dislocation and blood was collected from hearts and kept at 4°C for 24 h, centrifuged at 3000 rpm for 15 min, and serum was obtained as the supernatant.
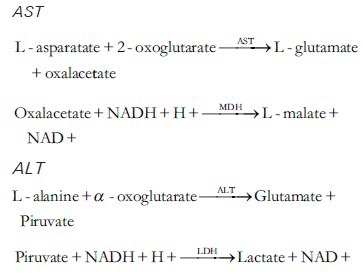
Determination of AST
Enzymatic determination was carried out in serum in optimal conditions of temperature and substrate and cofactor concentrations. Aspartate aminotransferase (AST) and Alanine aminotransferase (ALT) activity were determined in serum following the method of Rej and Horder.[10]
The activities of these enzymes were determined spectrophotometrically, by measuring the decrease in absorbance at 340 nm at 37°C, produced by the oxidation of NADH to NAD + in the coupled reaction of reduction of oxaloacetate to malate, catalyzed by malate dehydrogenase, according to the following process:
General
IR spectra measured in MeOH on a Perkin Elmer 2000 FT-IR spectrophotometer. Optical rotations were determined in MeOH on a Perkin Elmer 341 polarimeter. NMR measurements performed at 400 MHz for 1H and 100 MHz for13 C on a VARIAN 400 spectrometer from CDCl3, CD3OH, DMSO-d6 solutions. Column chromatography (CC) was carried out on Merck silica gel 60 (Aldrich, 230-400 mesh ASTM) and Sephadex LH-20 Sigma Aldrich TLC was carried on aluminum sheets with silica gel 60 F254 Merck, sprayed on with 5% methanolic FeCl3 and/ or 1% methanolicdiphenylboric acid-b-ethylamino ester.
Statistical analysis
The results were calculated as the means ± SD of four experimental observations in duplicate (four animals). Differences between groups were analyzed by an ANOVA following Snedecor F (α =0.05). Students’ test was performed for statistical evaluation as follows: (a) all values against their control; (b) differences between two groups Gs + TA versus TA.
RESULTS
Active compounds of Geranium schiedeanum
The phytochemical study of aqueous phase of Geranium schiedeanum led to the isolation of some polyphenolic compounds described as: (I) gallic acid, (III) acetonylgeraniin and (IV) ellagic acid, and lesser portion of kaempferol glycoside flavonoid (II) [Figures 2 and 3]. Is relevant to note that is the first time discloses the compound II in the Geranium genus and further that the yield of compound III in the crude extract was 54%.
Figure 2.
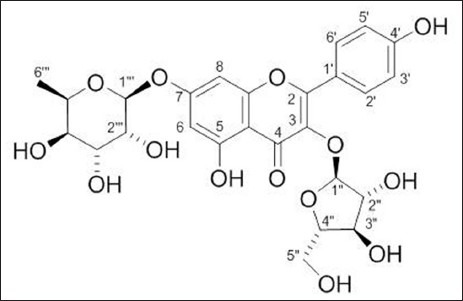
Compound (II) Kaempferol 3-O-α-L-arabinofuranoside-7-O-β-L-rhamnopyranoside
Figure 3.
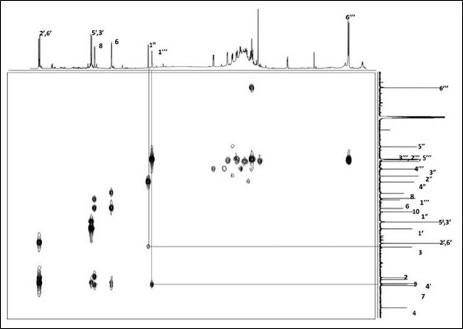
HMBC experiment of compound II
Kaempferol 3-O-α-L-arabinofuranσsyl-7-O-α-L-ramnopyranoside (II): Yellow amorphous powder; mp 190-192°C; mg (= −168° c = 0.0098 in MeOH); 1H NMR (400 MHz) DMSO-d6 : δ 8.09 (d, 2, J = 8.8 Hz, H-2’, H-6’), 6.92 (d, 2, J = 8.8 Hz, H-3’, H-5’), 6.84 (d, 1, J = 2.0 Hz, H-8), 6.46 (d, 1, J = 2.0 Hz, H-6), 5.66 (br s, 1, H-1”), 5.57 (d, 1, J = 1.5 Hz, H-1”’), 4.18 (br d, 1, J = 2.9 Hz, H-2”’), 3.86 (br s, 1, H-2”), 1.15 (d, 3, J = 6.36 Hz, H-6”’) 3C NMR (100 MHz) DMSO-d6: δ 178.9 (C-4), 162.7 (C-7), 161.9 (C-5), 161.2 (C-4’), 158.3 (C-2), 156.9 (C-9), 134.7 (C-3), 131.9 (C-2’, C-6’), 121.6 (C-1’), 116.5 (C-3’, C-5’), 109.1 (C-1”), 106.7 (C-10), 100.4 (C-6), 99.3 (C-1”’), 95.6 (C-8), 87.4 (C-4”), 83.1 (C-2”), 78.1 (C-3”), 72.6 (C-4”’), 71.3 (C-3”’), 71.1 (C-5”’), 70.8 (C-2”’), 61.9 (5”), 18.9 (C-6”’).
Acute oral toxicity study
The acetone-water extract of Geranium schideanum was found to be non-toxic because in both phases the treatment did not cause any deaths [Table 1]. Therefore, the estimated LD50 was above 5000 mg/kg.[11] The animals evaluated did not show any important changes in behavior, body weight or macroscopic morphology of heart, lung, liver and kidney compared with the control group. Based on Lorke method used to estimate the acute oral toxicity, LD50 values greater than 5000 mg/kg are of no practical interest, hence Gs extract was safe in this acute oral toxicity test.
Table 1.
DL50 estimation for the Gs extract using Lorke method
Aspartate aminotransferase and alanine aminotransferase
Liver damage induced by xenobiotics is characterized by the release in serum of hepatic enzymes due to necrosis of hepatocytes. ALT and AST is randomly distributed in the hepatic acinus, and is the enzyme activity used as marker of necrosis. The pre-treatment with the crude extract in the model of thioacetamide-induced hepatotoxicity in rats decreased and delayed markedly liver injury at 24 h [Figures 4 and 5]. That means that levels of ALT and AST were significantly lower in the rats pretreated with Gs extract. No effects were detected on serum activities when Gs was administered without thioacetamide (data not shown).
Figure 4.
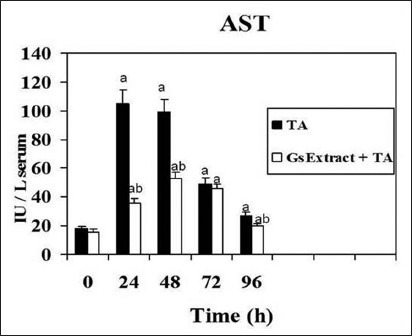
Effect of Gs pre-treatment on aspartate aminotransferase activity in serum of rats intoxicated with one sublethal dose of thioacetamide. Samples were obtained at 0, 24, 48, 72 & 96 h following thioacetamide (TA). The results, expressed as nmol per min per ml of serum, are the mean ± SD of four determinations in duplicate from four rats. Differences against the respective control are expressed as (a) and differences due to Gs extract are expressed as (b) P<0.05.
Figure 5.
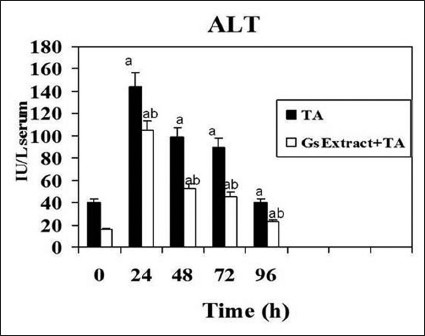
Effect of Gs pre-treatment on alanine aminotransferase activity in serum of rats intoxicated with one sublethal dose of thioacetamide. Samples were obtained at 0, 24, 48, 72 & 96 h following thioacetamide (TA). The results, expressed as nmol per min per ml of serum, are the mean ± SD of four determinations in duplicate from four rats. Differences against the respective control are expressed as (a) and differences due to Gs extract are expressed as (b) P<0.05.
DISCUSSION
Based on the results from the Lorke test, the oral LD50 value for Gs extract was above 5000 mg/kg, suggesting that it is practically nontoxic.
On the other hand, due liver is very susceptible to damage because it is exposed to a huge amount of substances, over the years various research with plant extracts has been proposed as models for their protection. The work done by Qureshi et al. with the extract from the leaves of Cordia macleodii in the model of liver injury induced by carbon tetrachloride (CCl4) showed a significant reduction in the levels of AST, ALT and total bilirubin, this effect was attributed to the content of flavonoids and triterpenoids of which have been found antioxidant[12,13] and hepatoprotective activity.[14]
In another experiment,[15] performed with the aqueous extract of MeOH extract of Phyllanthusniruri, using CCl4 as hepatotoxic agent, also was founded the hepatoprotective effect by decreasing the levels of ALT and AST. Moreover, in a recent study[16] with the same species showed significantly reduced liver damage in rats intoxicated with TAA; reported reduced levels of ALT, AST, ALP and BILT comparing results with the silymarin control and a substance isolated from the leaves of Silybum marinum hepatoprotective effect which has been previously studied. The increased activity of the enzymes ALT, AST and ALP in intoxicated rats compared to controls indicates the presence of necrosis of hepatocytes, resulting in deficiency of transaminase and constant release of ALP cholestasis probably attributed to causing structural alterations and the integrity of the hepatocyte.
There is evidence that free radicals play a critical role in certain pathological conditions such as some cancers, multiple sclerosis, inflammation, arthritis and arterosclerosis.[17] For this reason, some research objectives are directed toward the development or discovery of compounds catchers of these radicals. In the same way searching for antioxidants from plants has been of major interest of scientist in the last decades. However, scientific studies on antioxidant properties of wild plants are still rather scarce. The assessment of antioxidant activity of wild plants remains an interesting and useful task for finding new sources of natural products.[18]
One of the major components in Geranium species is geraniin[19] described by its discoverer as a crystallizable tannin. This substance first isolated from Geranium thunbergii Sieg. Et Zucc. by T. Okuda in 1976, has been evaluated showing an antihypertensive activity, also geraniin inhibits the angiotensin converting enzyme[20,21] and reverse transcriptase of tumoral viruses RNA,[22] inhibits HSV-1 and HSV-2 multiplication at different magnitudes of potency and also is an excellent antioxidant. But geraniin, is not a stable compound, and also is difficult to obtain, that is why, in the present study we used acetonylgeraniin a derivade of geraniin more stable and easily obtainable form acetone-water extract. Nevertheless, acetonylgeraniin, known as an artifact, wasn’t studied before.
A large number of plant species like G. schiedeanum contain chemical compounds that exhibit the ability to trap free radicals. The ability to trap free radicals has been called antioxidant activity. The phytochemical study of G. schiedeanum led to the isolation of hydrolysable tannins, well known as potent antioxidants: gallic acid, geraniin and ellagic acid and a lesser proportion of kaempferol glycoside flavonoid (3-O-α-L-arabinofuranoside-7-O-β-D-rhamnoside de Kaempferol), notably, is the first time discloses these compounds in the genus. Further the yield of geraniin in the crude extract was 40%.
The acute liver injury induced by a necrogenic dose of thioacetamide (TA), a potent hepatotoxic agent, is characterized by a severe perivenous necrosis. The necrosis develops as a consequence of the biotransformation of TA through the microsomal flavin-dependent monooxygenase.[23,24] The reactive metabolites responsible for TA hepatotoxicity are the radicals derived from thioacetamide-S-oxide and the reactive oxygen species derived as sub products in the process of microsomal TA oxidation. In the present study, TA-induced hepatotoxicity was used to investigate the effect of the pretreatment of G. schiedeanum on the events involved in liver regeneration. The results obtained in the present study provide evidence that Gs when administered intravenously prior to TA significantly reduces liver damage because the pre-treatment with the crude extract in the model of thioacetamide-induced hepatotoxicity in rats decreased and delayed liver injury by 66% at 24 hours.[25]
CONCLUSIONS
There is evidence that free radicals play a critical role in certain pathological conditions such as some cancers, multiple sclerosis, inflammation, arthritis and arterosclerosis. For this reason, some research objectives directed toward the development or discovery of these compounds catchers of these radicals. The data obtained indicate that the crude Gs extract pre-treatment has hepatoprotective and antioxidant effect in damage induced by TA. This result suggests that Gs extract may be used as an alternative for reduction of liver damage. However, further investigation on the mechanism of the hepatoprotective effect of the plant species needs to be carried out.
ACKNOWLEDGEMENTS
The authors would like to thank Teresa Vargas for her valuable technical Assistance. Supported by Grant of PROMEP-MEXICO UAEHGO-PTC-454.
Footnotes
Source of Support: None
Conflict of Interest: None declared.
REFERENCES
- 1.Pérez Escandón BE, Villavicencio MA, Ramirez Aguirre A. 1st ed. Méico: Universidad Autónoma del Estado de Hidalgo; UAEH; 1998. Lista floristica del estado de hidalgo recopilación Bibliografica. [Google Scholar]
- 2.Okuda T, Yoshida TY, Hatano TJ. Ellagitannins as active constituents of medicinal plants. Nat Prod. 1989;55:117–22. doi: 10.1055/s-2006-961902. [DOI] [PubMed] [Google Scholar]
- 3.Okuda T, Mori K, Hayashi N, Yoshida T, Mori K. Consitutents of Geranium Thunbergii er Zucc II. Ellagitannins. Phytochemistry. 1975;14:1877–8. [Google Scholar]
- 4.Camacho-L A, Gayosso-De-Lucio J, Torres-Valencia J, Muñoz.Sánchez J, Alarcon-Hernandez E, Rogelio L. Antioxidant Constituents of Geranium bellum Rose. J Mex Chem Soc. 2008;52:103–7. [Google Scholar]
- 5.Gayosso-De-Lucio JA, Torres-Valencia JM, Cerda-García-Rojas CM, Joseph-Nathan P. Ellagitannins from Geranium potentillaefolium and G. bellum. Nat Prod Comm. 2010;5:531–4. [PubMed] [Google Scholar]
- 6.Radulović NS, Stojković MB, Mitić SS, Randjelović PJ, Ilić IR, Stojanović NM, et al. Exploitation of the antioxidant potential of Geranium macrorrhizum (Geraniaceae): Hepatoprotective and antimicrobial activities. Nat Prod Commun. 2012;7:1609–14. [PubMed] [Google Scholar]
- 7.Bautista M, Andres D, Cascales M, Morales-González JA, Sánchez-Reus M. Effect of Gadolinium Chloride on Liver Regeneration Following Thioacetamide-Induced Necrosis in Rats. Int J Mol Sci. 2010;11:4426–40. doi: 10.3390/ijms11114426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sanz N, Diez-Fernández C, Andrés D, Cascales M. Hepatotoxicity and aging: Endogenous antioxidant systems in hepatocytes from 2-, 6-, 12-, 18- and 30-month-old rats following a necrogenic dose of thioacetamide. Biochim Biophys Acta. 2002;1587:12–20. doi: 10.1016/s0925-4439(02)00048-0. [DOI] [PubMed] [Google Scholar]
- 9.Zaragoza A, Andrés D, Sarrión D, Cascales M. Potentiation of thioacetamide hepatotoxicity by phenobarbital pretreatment in rats. Inducibility of FAD monooxygenase system and age effect. Chem Biol Interact. 2000;124:87–101. doi: 10.1016/s0009-2797(99)00147-7. [DOI] [PubMed] [Google Scholar]
- 10.Rej RY, Horder M. Aspartate aminotransferase. L-aspartate: 2-oxoglutarate aminotranferase, EC 2.6.2.1. Routine U.V. method. In: Bergmeyer HU, editor. Methods of Enzymatic Analysis. 3rd ed. Vol. 3. Weinheim: Verlag Chemie Publisher; 1984. pp. 416–24. [Google Scholar]
- 11.Lorke D. A New Approach to Practical Acute Toxicity Testing. Arch Toxicol. 1983;54:275–87. doi: 10.1007/BF01234480. [DOI] [PubMed] [Google Scholar]
- 12.Qureshi NN, Haleem MA. Antioxidant and hepatoprotective activity of Cordia macleodii leaves. Saudi Pharm J. 2009;17:299–302. doi: 10.1016/j.jsps.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tatsimo S, Tamokou J, Havyarimana L, Csupor D, Forgo P. Antimicrobial and antioxidant activity of kaempferolrhamnoside derivatives from Bryophyllumpinnatum. BMC Res Notes. 2012;5:158. doi: 10.1186/1756-0500-5-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shinno E, Shimoji M, Imaizumi N, Kinoshita S, Sunakawa H, Aniya Y. Activation of rat liver microsomal glutathione S-transferase by gallic acid. Life Sci. 2005;78:99–106. doi: 10.1016/j.lfs.2005.04.034. [DOI] [PubMed] [Google Scholar]
- 15.Srivastava A, Shivanandappa T. Hepatoprotective effect of the aqueous extract of the roots of Decalepishamiltonii against ethanol-induced oxidative stress in rats. Hepatol Res. 2006;35:267–75. doi: 10.1016/j.hepres.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 16.Amin ZA, Bilgen M, Alshawsh MA, Ali HM, Hadi AH, Abdulla MA. Protective Role of Phyllanthusniruri Extract against Thioacetamide-Induced Liver Cirrhosis in Rat Model. Evid Based Complement Alternat Med 2012. 2012:241583. doi: 10.1155/2012/241583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Latté KP, Kolodziej H. Antioxidant properties of phenolic compounds from Pelargonium reniforme. J Agric Food Chem. 2004;52:4899–902. doi: 10.1021/jf0495688. [DOI] [PubMed] [Google Scholar]
- 18.Cheng JT, Chang SS, Hsu FL. Antihypertensive action of geraniin in rats. J Pharm Pharmacol. 1994;46:469. doi: 10.1111/j.2042-7158.1994.tb03718.x. [DOI] [PubMed] [Google Scholar]
- 19.Wu N, Zu Y, Fu Y, Kong Y, Zhao J, Li X, et al. Antioxidant Activities and Xanthine Oxidase Inhibitory Effects of Extracts and Main Polyphenolic Compounds Obtained from Geranium sibiricum L. J Agric Food Chem. 2010;58:4737–43. doi: 10.1021/jf904593n. [DOI] [PubMed] [Google Scholar]
- 20.Kameda K, Takaku T, Okuda H, Kimura Y, Okuda T, Hatano T, et al. Inhibitory effects of various flavonoids isolated from leaves of persimmon on angiotensin-converting enzyme activity. J Nat Prod. 1987;50:680–3. doi: 10.1021/np50052a017. [DOI] [PubMed] [Google Scholar]
- 21.Ueno H, Horie S, Nishi Y, Shogawa H, Kawasaki M, Suzuki S, et al. Chemical and pharmaceutical studies on medicinal plants in Paraguay. Geraniin, an angiotensin-converting enzyme inhibitor from “paraparai mi,” Phyllanthus niruri. J Nat Prod. 1988;51:357–9. doi: 10.1021/np50056a033. [DOI] [PubMed] [Google Scholar]
- 22.Kakiuchi N, Hattori M, Namba T, Nisizahua M, Yamagishi T, Okuda TJ. Inhibitory effect of tannins on reverse transcriptase from RNA tumor virus. Nat Prod. 1985;48:614–21. doi: 10.1021/np50040a016. [DOI] [PubMed] [Google Scholar]
- 23.Fujiki H, Suganuma M, Kurusu M, Okabe S, Imayoshi Y, Taniguchi S, et al. New TNF-alpha releasing inhibitors as cancer preventive agents from traditional herbal medicine and combination cancer prevention study with EGCG and sulindac or tamoxifen. Mutat Res. 2003;523-524:119–25. doi: 10.1016/s0027-5107(02)00327-5. [DOI] [PubMed] [Google Scholar]
- 24.Cascales M, Martín-Sanz P, Craciunescu DG, Mayo I, Aguilar A, Robles-Chillida EM, et al. Alterations in hepatic peroxidation mechanisms in thioacetamide-induced tumors in rats. Effect of a rhodium complex. Carcinogenesis. 1991;12:233–40. doi: 10.1093/carcin/12.2.233. [DOI] [PubMed] [Google Scholar]
- 25.Dyroff MC, Neal RA. Studies of the mechanism of metabolism of thioacetamide-S-oxide by rat liver microsomes. Mol Pharmacol. 1981;41:3430–45. [PubMed] [Google Scholar]


