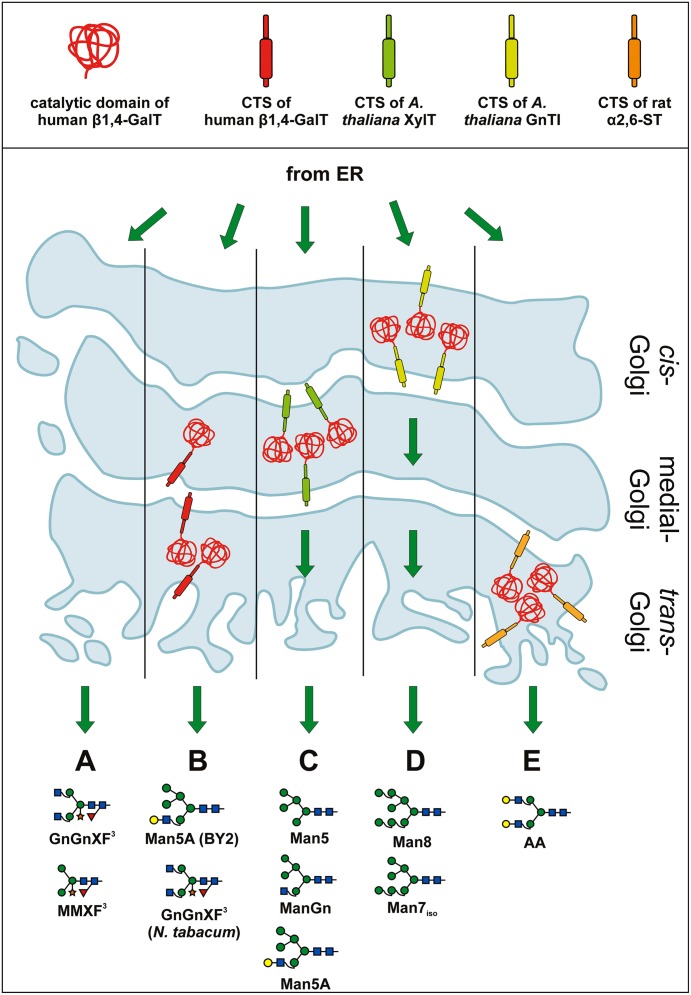
FIGURE 3.
Expression of β1,4-GalT and chimeric versions thereof in plants. Schematic presentation of various β1,4-GalT constructs expressed in plants and the consequences on the glycosylation profile of total and recombinantly expressed proteins. In the top panel β1,4-GalT catalytic domain and various CTS regions are illustrated in different colors. The color code is used to better visualize various chimeric fusion constructs. The bottom panel shows a Golgi stack and the hypothetical localization of different β1,4-GalT constructs. Green arrows indicate cargo flow from ER through the Golgi. Major glycan structures produced under the given conditions are shown. (A) Major glycoforms detected in wild-type plants (without the expression of β1,4-GalT) are complex N-glycans carrying xylose and fucose (i.e., GnGnXF, MMXF, etc.; e.g., Bakker et al., 2001). (B) Expression of full-length human β1,4-GalT in BY2 tobacco cells (Palacpac et al., 1999) and tobacco plants (Bakker et al., 2001) led to different results. In BY2 cells, mainly galactosylated, hybrid-type glycans (like Man5A) as well as oligomannosidic glycans were found (Palacpac et al., 1999). In tobacco plants (Bakker et al., 2001) GnGnXF remained the major glycoform and only small amounts of galactosylated glycans were found. These results indicate that β1,4-GalT acted in BY2 cells at an earlier stage of the glycosylation pathway than in tobacco plants, leading to interference with endogenous glycosylation reactions in cells, but not in plants. (C) Major glycoforms detected upon expression of a chimeric GalT, that carries the CTS region of A. thaliana β1,2-xylosyltransferase (indicated in pale green) and targets the enzyme to a medial stage of the glycan processing pathway: Man5, ManGn, Man5A. A drastically reduced amount of xylosylated and fucosylated glycans was detected (Bakker et al., 2006). The results point to an early activity of the chimeric β1,4-GalT, most probably in medial Golgi stacks. (D) Targeting the GalT to an even earlier compartment by fusing it to the CTS of the cis-Golgi acting GnTI (indicated in yellow; Vézina et al., 2009) induced the production of nearly exclusively oligomannosidic structures. Only minute amounts of galactosylated, hybrid Man5A were present. (E) Upon expression in a XT/FT knock-down plant line of a chimeric GalT carrying the late-Golgi CTS of rat α2,6-sialyltransferase (indicated in orange) proteins carrying mainly galactosylated glycans (e.g., AA) were generated (Strasser et al., 2009). These results indicate that the ST-GalT fusion is indeed located in a late Golgi stack where final N-glycan processing takes place.