Abstract
Background:
Buddlejasaponin IV (BS-IV), a triterpene saponin isolated from Pleurospermum kamtschaticum HOFFMANN (Umbelliferae), is known to have potent anti-inflammatory activity and cytotoxicity against diverse cancer cell lines. In the present study, we attempted to verify whether BS-IV could inhibit cell growth, and induce cell cycle arrest and apoptosis in highly invasive YD-10B human oral squamous cell carcinoma (OSCC) cells.
Methods:
YD-10B cells were treated with various concentrations of BS-IV, and the cell viability was evaluated by MTT assay. Flow cytometry was conducted to examine cell phase distribution and DAPI staining was performed to observe apoptotic morphological changes in BS-IV-treated YD-10B cells. Western blot analysis was used to investigate the expression of proteins associated with cell cycle arrest and apoptosis.
Results:
BS-IV treatment significantly reduced the viability of YD-10B cells and partially arrested cell cycle progression at the G2/M phase. Treatment with BS-IV substantially decreased the levels of cyclin B1 and stimulated the phosphorylation of checkpoint kinase 2 (Chk2). The expression of p21 was increased but the phosphorylation of Akt was inhibited in BS-IV-treated YD-10B cells. Furthermore, BS-IV induced release of cytochrome c from mitochondria by reducing anti-apoptotic Bcl-2 level and increasing pro-apoptotic Bax level. Active caspase-3 level and the cleavage of poly (ADP-ribose) polymerase (PARP) were enhanced by BS-IV treatment. In addition, BS-IV increased the expression of Fas death receptor and its ligand (FasL) in YD-10B cells.
Conclusions:
The treatment with BS-IV inhibits the growth of YD-10B cells by inducing p21-dependent cell cycle arrest at G2/M phase and apoptosis through both mitochondrial-dependent and death receptor-mediated pathways. Thus, BS-IV is an excellent candidate for a chemopreventive agent to block the progression of human OSCC.
Keywords: Oral squamous cell carcinoma, Buddlejasaponin IV, Cell cycle arrest, Apoptosis
INTRODUCTION
Oral squamous cell carcinoma (OSCC) is the most common malignant tumor of the oral cavity worldwide and consistently increasing in developing countries. OSCC is characterized by a high degree of local invasiveness and highly metastasize to the cervical lymph nodes.1,2 Despite recent improved clinical surgery and development of new anticancer drugs, OSCC has high mortality rate in patients with distant metastases. Therefore, the early diagnosis and standard treatments is extremely important to inhibit the onset and progression of oral carcinomas.3 Furthermore, alternative cancer therapy has been demanded for control OSCC and phytochemicals has been suggested as the major source for anticancer agents against a various malignant tumors.
Apoptosis, the programmed cell death, is critical for cellular homeostasis and is also one of the important mechanisms for the treatment of various cancers.4 The apoptotic process is induced by both death receptor (extrinsic) pathway and mitochondrial (intrinsic) pathway. The death receptor-mediated pathway involves the Fas, FasL and procaspase-8. The mitochondria-dependent pathway is controlled by members of Bcl-2 family, causing the release of cytochrome c from mitochondria and the activation of caspase cascade. Two main death pathways converge at caspase-3 activation, and then activated caspase-3 cleaves many substrate proteins including the DNA repair enzyme PARP.5
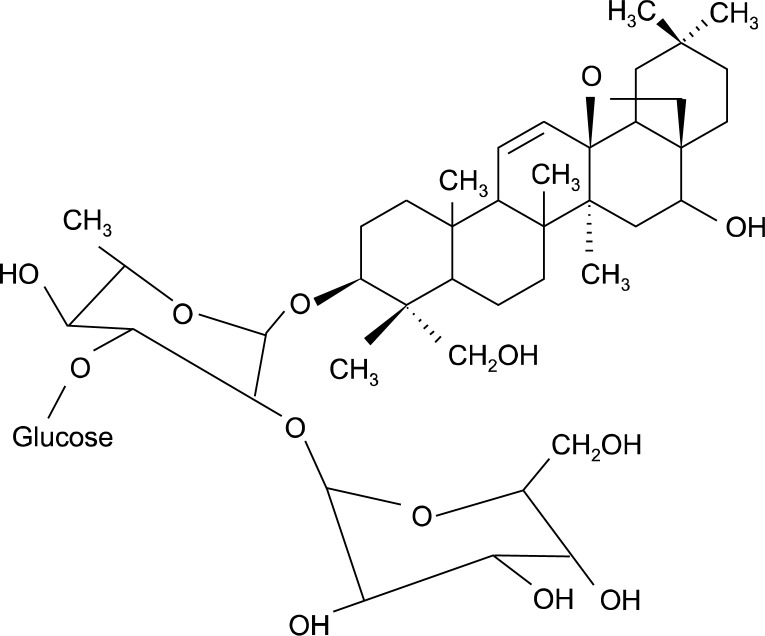
Buddlejasaponin IV (BS-IV) (Fig. 1) is a major component of the aerial part of Pleurospermum kamtschaticum Hoffmann (Umbelliferae), and has been reported as potent anti-inflammatory agent inhibiting the production of nitric acid, prostaglandin E2, tumor necrosis factor-α and interleukin (IL)-1β as well as the expression of inducible nitric oxide synthase and cyclooxygenase-2 in lipopoly-saccharide-stimulated RAW264.7 macrophages.6,7 BS-IV was also shown to evidence a remarkable hepatoprotective effect, antiviral activity and cytotoxicity against a panel of seven different cancer cell lines.8 In the previous study, we reported that BS-IV induces cell cycle arrest and apoptosis in human papillomaviruses infection-immortalized human oral keratinocytes.9
Fig. 1.
Chemical structure of BS-IV.
The present study aimed to estimate the chemopreventive potential of BS-IV against the highly invasive human OSCC cell line. We examined whether BS-IV could induce cell cycle arrest and apoptosis in YD-10B cells, and further explored the mechanism underlying its activity.
MATERIALS AND METHODS
1. Materials
BS-IV was generously provided by Professor Hee-Juhn Park in Sangji University,6 dissolved in dimethyl sulfoxide (DMSO), stored at −20°C and further diluted with the culture medium. Highly invasive YD-10B human OSCC cells, which have been derived from tongue cancer tissues of patients, were obtained by Professor Jin Kim in Yonsei University College of Dentistry.10 DMEM: nutrient mixture F-12 (DMEM/F-12), fetal bovine serum (FBS), antibiotic-antimycotic (10,000 units/ml penicillin G sodium, 10,000 μg/ml streptomycin sulfate and 25 μg/ml amphotericin B), phosphate-buffered saline (PBS), and 0.25% trypsin-EDTA were purchased from Gibco BRL (Rockville, MD, USA). Cholera toxin, hydrocortisone, insulin, transferrin, triiodothronine (T3), 3-(4,5-Dimethylthiazol-2-yl) 2,4-diphenyl tetrazolium bromide (MTT), RNase-A, Tween 20, DMSO, propidum iodide (PI), 4’,6-diamidino-2-phenylindole (DAPI) and phenylmethylsulfonylfluoride (PMSF) were purchased from Sigma-Aldrich (St. Louis, MO, USA). The following antibodies were purchased from their respective sources: Bax, Bcl-2, PARP and caspase-3 (Cell Signaling Technology, Denver, MA, USA); GAPDH, cytochrome c, Fas, FasL, cyclin B1, Cdc2, Cdk2, Cdc25C, Chk2, phospho-Chk2, p21, Akt, and phospho-Akt (Santa Cruz Biotechnology, Santa Cruz, CA, USA); horseradish peroxidase (HRP)-conjugated secondary antibodies (Amersham Life Science, Little Chalfont, UK).
2. Cell culture
YD-10B OSCC cells were grown in DMEM/F12 containing 10% FBS, 1×10−10 M cholera toxin, 0.4 mg/ml hydro-cortisone, 5 μg/ml insulin, 5 μg/ml transferrin and 2×10−11 M T3 at 37°C in a humidified atmosphere containing 5% CO2.
3. MTT assay
YD-10B cells (5×103 cells/well) were plated into a 96-well culture plate, and left overnight to adhere. The attached cells were treated with various concentrations of BS-IV for 24 and 48 h, respectively. Viable cells were detected using a 5 mg/ml MTT solution for an additional 4 h at 37°C, followed by dissolving the produced formazan product in cells with 200 μl DMSO. Absorbance was measured at 570 nm using a Benchmark microplate reader (Bio-Rad, Hercules, CA, USA).
4. Cell cycle analysis
YD-10B cells (4×105 cells/well) were treated with 10 μM BS-IV for 24 h. The cells were harvested, washed in cold PBS, fixed in 70% ethanol and stored at 4°C for 2 h. For DNA content analysis, the cells was treated with 0.25 mg/ml RNase-A for 30 min and stained with 50 μg/ml PI in 1.12% sodium citrate at room temperature. DNA content was analyzed by FACSCalibur using WinMDI 2.8 software (BD, Franklin Lakes, NJ, USA).
5. Western blot analysis
YD-10B cells were treated with different concentrations of BS-IV for 24 h. The harvested cells were suspended in lysis buffer containing 50 mM Tris (pH 7.5), 1% NP-40, 2 mM EDTA, 10 mM NaCl, 20 μg/ml aprotinin, 20 μg/ml leupeptin and 1 mM PMSF, and placed on ice for 30 min. After centrifugation at 12,000×g for 30 min at 4°C, the supernatant was collected, and then the protein concentration in the supernatant was determined with a Bradford protein assay kit. Equal amounts of protein (50 μg) were loaded, separated on 10% SDS-polyacrylamide gels and transferred onto a polyvinylidene difluoride membrane (Millipore, Billerica, MA, USA). The membrane was blocked with 5% skim milk in PBS containing 0.1% Tween-20 and then incubated with specific primary antibody (1:1,000) against each protein overnight at 4°C. The blots were incubated with a 1:3,000 dilution of the respective HRP-conjugated secondary antibody for 2 h at room temperature. The target proteins were visualized with an enhanced chemiluminescence detection kit according to the protocol of the manufacturer (Amersham Life Science).
6. DAPI staining
YD-10B cells (1×103 cells/well) were treated with 10 μM BS-IV for 24 h, washed twice with PBS and fixed in 4% paraformaldehyde. The cells were then stained with 1 μg/ml DAPI solution for 10 min at room temperature. The condensed or fragmented nuclei in apoptotic cells were observed under a fluorescence microscope (Olympus, Tokyo, Japan).
7. Cytochrome c release
YD-10B cells, treated with BS-IV, were harvested and suspended in ice-cold buffer A (20 mM HEPES, 250 mM sucrose, 10 mM KCl, 1.5 mM MgCl2, 1 mM EDTA, 1 mM EGTA, 1 mM DTT, 1 mM PMSF, 10 μg/ml aprotinin and 5 μg/ml leupeptin, pH 7.5). The cells were passed through 26-gauge needle 10 times, and then centrifuged at 750×g for 10 min at 4°C. The supernatant was twice centrifuged at 12,000×g for 15 min 4°C, and the resulting supernatant was collected as the cytosolic fraction. The cytosolic fraction (50 μg protein) was electrophoresed on 12% SDS-polyacrylamide gels and analyzed by western blotting with a specific antibody against cytochrome c.
8. Statistical analysis
Data were expressed as the means±standard deviation (SD) of three independent experiments and analyzed via one-way ANOVA with multiple comparisons using InStatTM statistical software (GraphPad Software, Inc., San Diego, CA, USA). P values of less than 0.05 were considered statistically significant.
RESULTS
1. BS-IV inhibits the viability of YD-10B OSCC cells
When YD-10B human OSCC cells were cultured in serum-free medium with various concentrations of BS-IV, cell viability was dose- and time-dependently decreased (Data not shown). Its half maximal inhibitory concentration (IC50) values were 22.5 μM for 24 h treatment and 9.6 μM for 48 h treatment.
2. BS-IV induces cell cycle arrest at G2/M phase in YD-10B OSCC cells
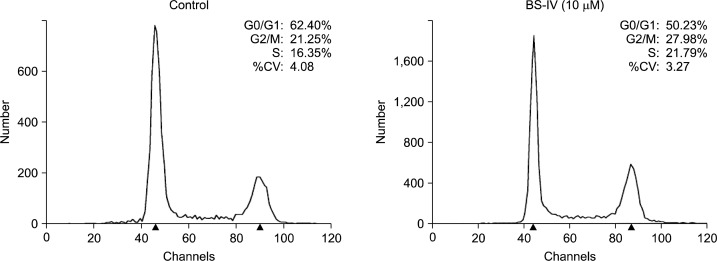
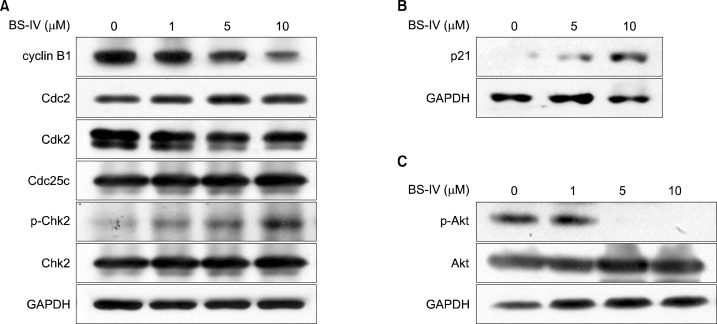
We determined whether BS-IV could block cell cycle progression of YD-10B cells by flow cytometric analysis. When YD-10B cells were treated with 10 μM BS-IV for 24 h, the percentage of YD-10B cells in the G2/M and S phase was slightly increased, but that in G0/G1 phase was decreased (Fig. 2). The cell cycle machinery is regulated by cyclins and Cdks, and the activities of the different cyclin-Cdk complexes modulate to control specific phases in the cell cycle. G2 to M phase progression is mainly controlled by the Cdc2-cyclin B1 complex, and Cdc25C phosphatase results in activation of Cdc2 by dephosphorylation.11 In addition, the phosphorylation of Chk2 activates the G2 checkpoint.12,13 Western blot analysis indicated that the levels of cyclin B1 and Cdk2 were significantly decreased and the level of the phosphorylated Chk2 was dose-dependently enhanced in BS-IV-treated YD-10B cells (Fig. 3A). BS-IV treatment did not affect the levels of Cdc2 and Cdc25c. In addition, the increased p21 protein level (Fig. 3B) and the reduced level of phosphorylated Akt (Fig. 3C), which can directly phosphorylate p21 and drop its level in nucleus,14 were observed in YD-10B cells treated with BS-IV for 24 h.
Fig. 2.
BS-IV suppressed cell cycle progression in YD-10B cells. Cells were treated with 10 M BS-IV for 24 h, fixed in 70% ethanol and the nuclei were stained with PI. The percentages of cell phase distribution were analyzed by flow cytometry. Data are the representative of three independent experiments.
Fig. 3.
BS-IV regulated the expression of G2/M regulatory proteins in YD-10B cells. Cells were treated with the various doses of BS-IV for 24 h and total cell lysates were prepared. Cell cycle-related proteins (A), p21 (B), p-Akt and Akt (C) were analyzed by western blotting with the specific antibodies. Data are the representative of three independent experiments.
3. BS-IV induces apoptosis in YD-10B OSCC cells
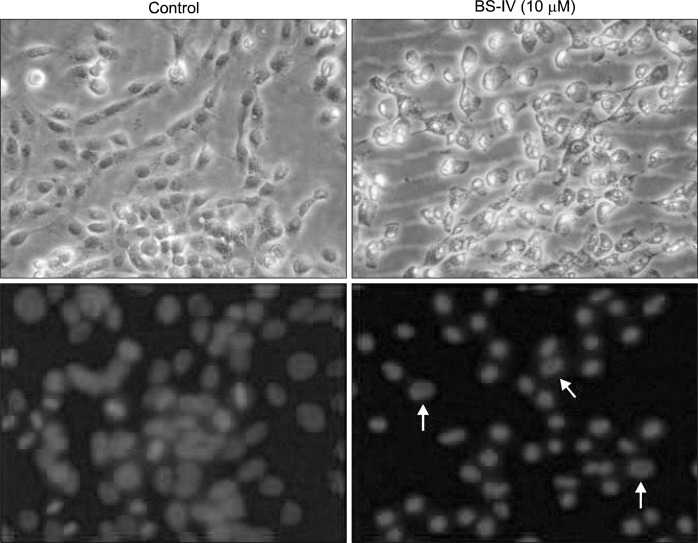
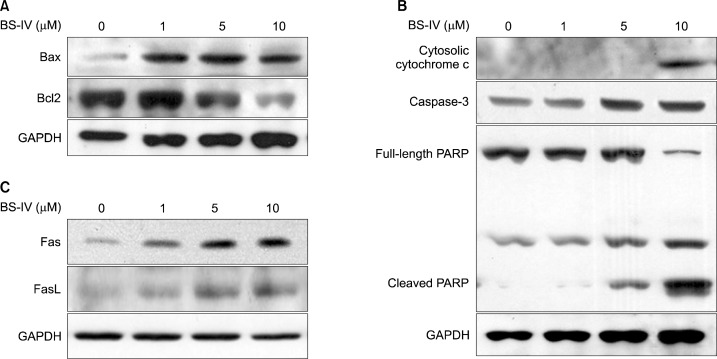
We further studied whether BS-IV could induce apoptosis in YD-10B cells. BS-IV treatment for 24 h induced morphological changes and apoptotic morphological changes were confirmed by DAPI staining (Fig. 4). The expression of Bax was increased and Bcl-2 level was reduced in BS-IV-treated cells (Fig. 5A). Consequently, BS-IV treatment resulted in an increase in the Bax/Bcl-2 ratio, leading to the release of cytochrome c from mitochondria (Fig. 5B). Moreover, treatment with BS-IV elevated the level of active caspase-3 and induced the cleavage of PARP (Fig. 5B). Furthermore, BS-IV stimulation caused the increased expression of Fas and FasL (Fig. 5C).
Fig. 4.
BS-IV stimulation induced apoptotic morphological change of YD-10B cells. Cells were treated with 10M BS-IV for 24 h and stained with 1 μg/ml DAPI solution. The nuclei in apoptotic cells were observed under a fluorescence microscope. The morphology of cells was observed under bright field microscopy. Upper panel showed the cells under bright field microscope, lower panel showed the cells under fluorescence microscope (×400 original magnification). Data are the representative of three independent experiments.
Fig. 5.
BS-IV regulated apoptotic proteins in YD-10B cells. Cells were treated with 1, 5 or 10 μM of BS-IV for 24 h. Total lysates and cytosolic fractions were prepared and electrophoresed on 10–12% SDS-polyacrylamide gel. The expression of Bax and Bcl2 (A), cytosolic cytochrome c, caspase-3 and PARP (B), Fas and FasL (C) was detected by western blotting using specific antibodies. The data are representative of three independent experiments.
DISCUSSION
OSCC is particularly dangerous because of a high risk of producing secondary tumors and captures about 90% of overall oral cancers.2 Combination therapy of surgical and non-surgical approaches has been developed, but there has been no significant decline in the mortality rate over 20 years due to a lack of markers for early prognosis and the failure of advanced tumors to respond to chemotherapy. In this study, we estimate whether BS-IV can be a promising candidate as a chemoprotective agent on OSCC. Human YD-10B cells are moderately differentiated squamouse cell carcinoma originated from tongue. YD-10B was characterized as OSCC cells showing a sheet of polygonal cells scattered with dyskeratotic cells and has high tumori-genicity and invasiveness. YD-10B cells typically expressed E-cadherin and EGFR, but did not express p53.15
Cell growth is controlled by the rates of cell proliferation and cell death. In most cancer cells, abnormal proliferation is induced, but cell death and differentiation are inhibited. Therefore, the manipulation of the apoptotic process in cancer cells is a critical point for the removal of transformed cells and preventing carcinogenesis. To elucidate YD-10B cell growth inhibition by BS-IV treatment, we investigated its effect on cell cycle progression and apoptosis. As a result, cell cycle was arrested at G2/M phase in BS-IV-treated YD-10B cells.
Next, we determined the molecular mechanism underlying cell cycle arrest and induction of apoptosis by BS-IV. Of cyclins and Cdks initiating entrance into M phase, BS-IV reduced expression of cyclin B1 and also increased the activation of Chk2, which are activated by ATM/ATR in response to DNA damage.16 Cyclin-Cdk complexes are regulated by Cdk inhibitors. Thus, the deregulated actions of the Cdk inhibitors contribute importantly to cancer development. In particular, p21 is targeted specifically to Cdc2 and Cdk2 complexes and regulated by Akt.17 Akt phosphorylates p21 Cdk inhibitor in the nucleus and exports them into the cytoplasm. Cytoplasmic p21 proteins do not influence cyclin-Cdk complexes.14 BS-IV treatment induced p21 expression and suppressed the phosphorylation of Akt. These findings demonstrate that treatment with BS-IV may at least inhibit cell growth by cell cycle arrest at G2/M phase due to the reduced expression of cyclin B1 and Chk2 activation. BS-IV-induced cell cycle arrest is closely associated with p53-independent p21 expression.
Apoptosis can be induced via the activation of the pro-apoptotic members of Bcl-2 family of proteins. In mitochondrial-dependent pathway, the increased pro-apoptotic Bcl-2-related proteins, particularly Bax, which antagonize the anti-apoptotic Bcl-2, favor cytochrome c release into cytosol through opening of the outer mito-chondrial membrane.18 The released cytochrome c results in the activation of the caspase cascade and resultant cleavage of PARP for DNA repair.19 We found that BS-IV induced release of cytochrome c from mitochondria by reducing anti-apoptotic Bcl-2 level and increasing pro-apoptotic Bax level. In turn, active caspase-3 and PARP cleavage were significantly increased in BS-IV-treated YD-10B cells. Furthermore, BS-IV suppressed the expression of Fas death receptor and FasL. These results suggest that BS-IV may induce apoptosis by two distinct signaling pathways via mitochondria and death receptor in YD-10B cells.
Collectively, the treatment with BS-IV inhibits the growth of YD-10B cells by inducing p21-dependent G2/M cell cycle arrest and apoptosis through both mitochondrial-dependent and death receptor-mediated pathways. BS-IV is an excellent candidate for a chemopreventive agent to block the progression of highly invasive OSCC. In addition, BS-IV has anti-inflammatory, anti-viral and anti-hepatotoxic activities as active compound of P. kamtschaticum traditionally used to treat various kinds of disorders such as colds, arthritis, and impotence. Thus, BS-IV and the extracts with it may serve as beneficial supplement for health promotion. Furthermore, the evaluation on bioavailability of BS-IV is determined.
Acknowledgments
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2009-0094027).
REFERENCES
- 1.Gil Z, Carlson DL, Boyle JO, Kraus DH, Shah JP, Shaha AR, et al. Lymph node density is a significant predictor of outcome in patients with oral cancer. Cancer. 2009;115:5700–10. doi: 10.1002/cncr.24631. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA: a Cancer Journal for Clinicians. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 3.Massano J, Regateiro FS, Januario G, Ferreira A. Oral squamous cell carcinoma: review of prognostic and predictive factors. Oral Surgery, Oral Medicine, Oral Pathology, Oral RadioLogy, and Endodontics. 2006;102:67–76. doi: 10.1016/j.tripleo.2005.07.038. [DOI] [PubMed] [Google Scholar]
- 4.Fisher DE. Apoptosis in cancer therapy: crossing the threshold. Cell. 1994;78:539–42. doi: 10.1016/0092-8674(94)90518-5. [DOI] [PubMed] [Google Scholar]
- 5.Chang YC, Huang KX, Huang AC, Ho YC, Wang CJ. Hibiscus anthocyanins-rich extract inhibited LDL oxidation and oxLDL-mediated macrophages apoptosis. Food and CheMiCal Toxicology: an International Journal Published for the British Industrial Biological Research Association. 2006;44:1015–23. doi: 10.1016/j.fct.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 6.Jung HJ, Kim SG, Nam JH, Park KK, Chung WY, Kim WB, et al. Isolation of saponins with the inhibitory effect on nitric oxide, prostaglandin E2 and tumor necrosis factor-alpha production from Pleurospermum kamtschaticum. Biological & Pharmaceutical Bulletin. 2005;28:1668–71. doi: 10.1248/bpb.28.1668. [DOI] [PubMed] [Google Scholar]
- 7.Won JH, Im HT, Kim YH, Yun KJ, Park HJ, Choi JW, et al. Anti-inflammatory effect of buddlejasaponin IV through the inhibition of iNOS and COX-2 expression in RAW 264.7 macrophages via the NF-kappaB inactivation. British Journal of Pharmacology. 2006;148:216–25. doi: 10.1038/sj.bjp.0706718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tundis R, Bonesi M, Deguin B, Loizzo MR, Menichini F, Conforti F, et al. Cytotoxic activity and inhibitory effect on nitric oxide production of triterpene saponins from the roots of Physospermum verticillatum (Waldst & Kit) (Apiaceae) Bioorganic & Medicinal Chemistry. 2009;17:4542–7. doi: 10.1016/j.bmc.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 9.Hwang YS, Chung WY, Kim J, Park HJ, Kim EC, Park KK. Buddlejasaponin IV induces cell cycle arrest at G2/M phase and apoptosis in immortalized human oral keratinocytes. Phytotherapy Research: PTR. 2011;25:1503–10. doi: 10.1002/ptr.3406. [DOI] [PubMed] [Google Scholar]
- 10.Lee HJ, Guo HY, Lee SK, Jeon BH, Jun CD, Lee SK, et al. Effects of nicotine on proliferation, cell cycle, and differentiation in immortalized and malignant oral keratinocytes. Journal of oral pathology & medicine: official publication of the International Association of Oral Pathologists and the American Academy of Oral Pathology. 2005;34:436–43. doi: 10.1111/j.1600-0714.2005.00342.x. [DOI] [PubMed] [Google Scholar]
- 11.O’Connor PM, Ferris DK, Hoffmann I, Jackman J, Draetta G, Kohn KW. Role of the cdc25C phosphatase in G2 arrest induced by nitrogen mustard. Proceedings of the National Academy of Sciences of the United States of America. 1994;91:9480–4. doi: 10.1073/pnas.91.20.9480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abraham RT. Cell cycle checkpoint signaling through the ATM and ATR kinases. Genes & Development. 2001;15:2177–96. doi: 10.1101/gad.914401. [DOI] [PubMed] [Google Scholar]
- 13.Niida H, Nakanishi M. DNA damage checkpoints in mammals. Mutagenesis. 2006;21:3–9. doi: 10.1093/mutage/gei063. [DOI] [PubMed] [Google Scholar]
- 14.Zhou BP, Liao Y, Xia W, Spohn B, Lee MH, Hung MC. Cytoplasmic localization of p21Cip1/WAF1 by Akt-induced phosphorylation in HER-2/neu-overexpressing cells. Nature Cell Biology. 2001;3:245–52. doi: 10.1038/35060032. [DOI] [PubMed] [Google Scholar]
- 15.Lee EJ, Kim J, Lee SA, Kim EJ, Chun YC, Ryu MH, et al. Characterization of newly established oral cancer cell lines derived from six squamous cell carcinoma and two mucoe-pidermoid carcinoma cells. Experimental & Molecular MeDiCine. 2005;37:379–90. doi: 10.1038/emm.2005.48. [DOI] [PubMed] [Google Scholar]
- 16.Huang H, Hu M, Zhao R, Li P, Li M. Dihydromyricetin suppresses the proliferation of hepatocellular carcinoma cells by inducing G2/M arrest through the Chk1/Chk2/Cdc25C pathway. Oncology Reports. 2013;30:2467–75. doi: 10.3892/or.2013.2705. [DOI] [PubMed] [Google Scholar]
- 17.Li Y, Dowbenko D, Lasky LA. AKT/PKB phosphorylation of p21Cip/WAF1 enhances protein stability of p21Cip/WAF1 and promotes cell survival. The Journal of Biological CheMistry. 2002;277:11352–61. doi: 10.1074/jbc.M109062200. [DOI] [PubMed] [Google Scholar]
- 18.Shimizu S, Narita M, Tsujimoto Y. Bcl-2 family proteins regulate the release of apoptogenic cytochrome c by the mitochondrial channel VDAC. Nature. 1999;399:483–7. doi: 10.1038/20959. [DOI] [PubMed] [Google Scholar]
- 19.Satoh MS, Lindahl T. Role of poly(ADP-ribose) formation in DNA repair. Nature. 1992;356:356–8. doi: 10.1038/356356a0. [DOI] [PubMed] [Google Scholar]