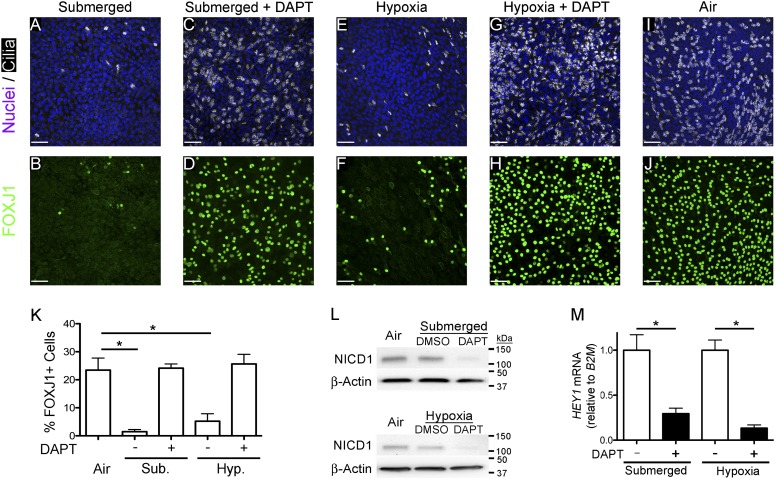
Figure 3.
γ-secretase inhibition restores FOXJ1 expression and ciliated cell differentiation in submerged and hypoxic conditions. (A–J) Representative extended-focus confocal immunofluorescent images of NHBE cells after 21 days of culture (A and B) submerged (0.5 ml), (C and D) submerged (0.5 ml) with 10 μM N-[(3,5-difluorophenyl)acetyl]-L-alanyl-2-phenyl]glycine-1,1-dimethylethyl ester (DAPT), (E and F) ALI in hypoxia (0.5% O2), (G and H) ALI in hypoxia (0.5% O2) with 10 μM DAPT, or (I and J) ALI in air, and stained for nuclei (Hoechst, blue) and cilia (acetylated α-tubulin, white) or FOXJ1 (green). Scale bars, 50 μm. (K) Graph displaying quantification of the percent FOXJ1+ in each condition showing a significant decrease in submerged and hypoxic conditions compared with the air control, and showing that DAPT restores ciliogenesis to control ALI levels. One-way ANOVA (*P < 0.05; n = 3). (L) Western blots of whole-cell lysates (40 μg) from NHBE cells cultured 24 hours at an ALI in air, submerged (0.5 ml) with and without 10 μM DAPT (top panel), or 48 hours at an ALI in air, and ALI in hypoxia (0.5% O2) with and without 10 μM DAPT (bottom panel) probed for activated-Notch1 (Notch intracellular domain [NICD] 1). The data show that NICD1 levels do not increase over ALI (Air), and that DAPT decreases NICD1 protein levels in submersion and hypoxia. β-actin was used as the loading control. (M) Quantitative RT-PCR (qRT-PCR) Notch target gene, hairy/enhancer-of-split related with YRPW motif 1 (HEY1), mRNA from NHBE cells cultured for 21 days in submerged and ALI in hypoxia (0.5% O2) (Hyp.) with and without 10 μM DAPT. HEY1 mRNA was normalized to the housekeeping gene, β2-microglobin (B2M), and shows that DAPT inhibits Notch signaling. Data shown are means (± SEM). Student’s t test (*P < 0.05; n = 3).