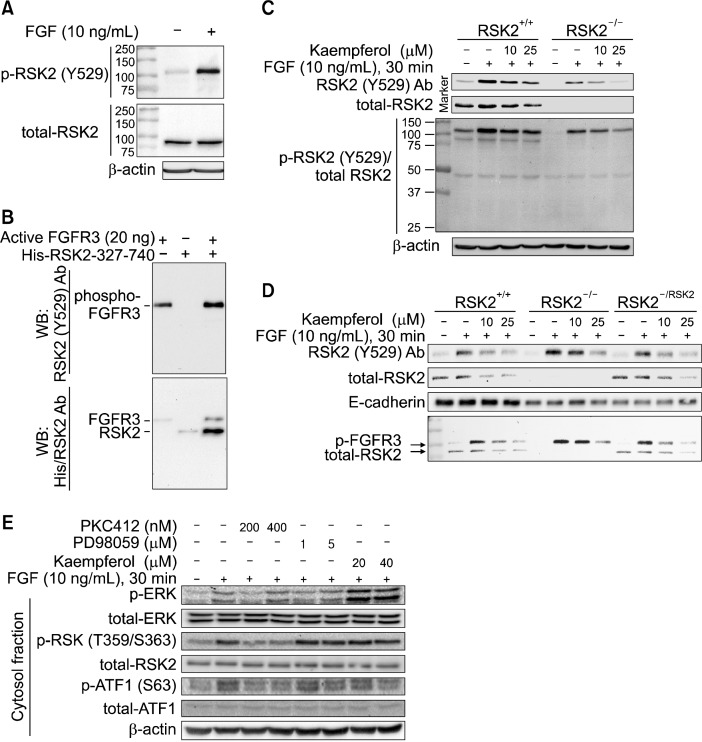
Figure 2.
Kaempferol inhibits FGFR 3 kinase activity. (A) phospho-RSK2 TyrY529 antibody recognizes a protein having different molecular weight from the RSK2. The JB6 Cl41 cells were stimulated with fibroblast growth factor (FGF) and membrane fraction proteins were extracted. The proteins were hybridized as indicated using phospho-RSK2 TyrY529 or total-RSK2 antibodies, and then horseradish peroxidase (HRP)-conjugated secondary antibody. β-Actin was used as an internal control to verify the equal protein loading. (B) phospho-RSK2 Tyr529 antibody recognizes FGFR3, but not RSK2. Purified His-RSK2-327-740 from Escherichia coli and active FGFR3 kinase were combined and carried out an in vitro kinase assay. The proteins were divided equally and indicated proteins were visualized by Western blotting using phospho-RSK2 Tyr529 and/or His-specific antibodies, and then HRP-conjugated secondary antibody. (C) Kaempferol inhibits FGFR3 phosphorylation. RSK2+/+ and RSK2−/− mouse embryonic fibroblasts (MEFs) (1.5 × 106) were seeded into 100-mm culture dishes, starved and stimulated with FGF and/or kaempferol as indicated. The membrane proteins were extracted, hybridized with phospho-RSK2 Tyr529 and total RSK2 antibodies as indicated, and visualized by Western blotting using HRP-conjugated secondary antibody. β-actin was used as an internal control to verify the equal protein loading. (D) Kaempferol inhibits FGFR3 phosphorylation and total RSK2 protein level. The pBabe-puro-RSK2 was infected into RSK2−/− MEFs and membrane proteins were extracted. The phospho-FGFR3 and total RSK2 proteins were visualized by Western blotting using specific antibodies as indicated. E-cardherin was used as an internal control to verify the equal protein loading of membrane fraction proteins. (E) Signaling profiles of FGFR3/RSK2 signaling axis by FGF stimulation. JB6 Cl41 cells were stimulated starved and stimulated with FGF with combination of chemical inhibitors as indicated. The membrane and cytosolic fraction proteins were extracted, and each protein was visualized by Western blotting using specific antibodies as indicated. β-Actin was used as an internal control to verify the equal protein loading.