Abstract
Background:
Carnosic acid, which is one of extract components of rosemary, has anti-inflammatory, anti-oxidant, and anti-cancer effects. However, the anti-cancer effect of carnosic acid in human renal carcinoma cells is unknown.
Methods:
Flow cytometry analysis was used to examine the effects of carnosic acid on apoptosis, and Asp-Glu-Val-Asp-ase activity assay kit was used to investigate the involvement of caspase activation. To determine protein expression of apoptotic and endoplasmic reticulum (ER) stress-related proteins, we used Western blotting. Intracellular accumulation of reactive oxygen species (ROS) was determined using the fluorescent probes 2’, 7’-dichlorodihydrofluorescein diacetate (H2DCFDA).
Results:
Carnosic acid induced sub-diploid DNA content, sub-G1, population and poly (ADP-ribose) polymerase (PARP) cleavage and activated caspase-3. A pan-caspase inhibitor, a benzyloxycarbonylvalyl-alanyl-aspartyl fluoromethyl ketone, markedly reduced apoptosis in carnosic acid-treated cells. Carnosic acid promoted intracellular ROS production, and pretreatment with the ROS scavengers (N-acetyl-L-cysteine and glutathione ethyl ester) inhibited carnosic acid-induced apoptosis. Furthermore, carnosic acid also induced expression of ER stress marker proteins, including activating transcription factor 4 (ATF4) and CCAAT/enhancer-binding protein-homologous protein (CHOP), in a dose- and time-dependent manner. Down-regulation of ATF4 and CHOP by small interfering RNA (siRNA) markedly reduced carnosic acid-induced sub-G1 population and PARP cleavage. In addition, carnosic acid induced apoptosis in human breast carcinoma MDA-MB-361 and human hepatocellular carcinoma SK-HEP1 cells, but not in normal human skin fibroblast cells and normal mouse kidney epithelial TMCK-1 cells.
Conclusion:
Carnosic acid induced apoptosis through production of ROS and induction of ER stress in human renal carcinoma Caki cells.
Keywords: Carnosic acid, Reactive oxygen species, Activating transcription factor 4, CCAAT/enhancer-binding protein-homologous protein
INTRODUCTION
Carnosic acid, which is a diterpene present in rosemary (Rosmarinus officinalis), has anti-tumor effects in in vitro, including inhibition of angiogenesis, proliferation, and migration. Carnosic acid inhibited vascular endothelial growth factor expression, which is an angiogenic marker, during 7,12-dimethylbenz[a]anthracene-induced hamster buccal pouch carcinogenesis.1 One of important anti-cancer effects induced by carnosic acid is inhibition of proliferation. Carnosic acid inhibited proliferation of leukemia cells and induced growth arrest at G2/M phase via down-regulation of cyclin A expression in colon cancer cells.2–4 In addition, carnosic acid reduced migration capacity through inhibition of urokinase plasminogen activator and metalloproteinases in colon cancer and melanoma cells.5,6 Furthermore, carnosic acid increased apoptosis in multiple cancer cells. For examples, carnosic acid induced apoptosis through inhibition of Akt and nuclear factor-κB signaling by induction of protein phosphatase 2A activity in prostate cancer cells.7 In neuroblastoma cells, carnosic acid also induced apoptosis by reactive oxygen species (ROS)-induced p38 mitogen-activated protein kinases activation.8 However, the effects of carnosic acid on apoptosis in renal carcinoma cells have been unknown.
The endoplasmic reticulum (ER) is a critical organelle to localize and fold of proteins in the cells, and a misbalance of homeostasis in ER is triggered by various stimuli, including hypoxia, glucose and calcium store depletion, and disulfide bonds reduction, followed by accumulation of unfolded and misfolded proteins in the lumen.9–12 Accumulation of unfolded and misfolded proteins in the ER induced cellular stress and elicited the unfolded protein response by Inositol Requiring Enzyme 1, RNA-dependent protein kinase-like ER kinase, and Activating Transcription Factor 6.13,14 If ER stress was induced in the cells, unfolded protein response promotes cellular responses to maintain cellular homeostasis, but if adaptation response is not sufficient, apoptosis was triggered.15,16 Multiple proteins are involved in ER stress-mediated apoptosis. Among them, activating transcription factor 4 (ATF4) has important roles in ER stress-mediated apoptosis. Anacardic acid increased ER stress-mediated apoptosis, and down-regulation of ATF4 suppressed apoptosis in hepatoma and melanoma cells, and bortezomib- or fenretinide-induced apoptosis is associated with ATF4 induction in human neuroectodermal tumor cells.17,18 In addition, CCAAT/enhancer-binding protein-homologous protein (CHOP) is also important for ER stress-mediated apoptosis. CHOP is involved in 15-Deoxy-Δ12,14-prostaglandin J2-mediated apoptosis in cervical cancer cells and rottlerin-induced apoptosis in colon cancer cells.19,20
In this study, we investigated whether carnosic acid induced apoptosis, and identified the molecular targets of carnosic acid-mediated apoptosis in human renal carcinoma Caki cells.
MATERIALS AND METHODS
1. Cell culture and materials
Human renal carcinoma (Caki), human hepatocellular carcinoma (SK-HEP1), and human breast carcinoma (MDA-MB-361) cells were obtained from the American Type Culture Collection (Manassas, VA, USA). The mouse kidney cells (TMCK-1) and the normal human skin fibroblasts were gifts from Dr. T.J. Lee (Yeungnam University, Korea). The culture medium used throughout these experiments was Dulbecco’s modified Eagle’s medium containing 10% fetal bovine serum, 20 mM N-2-Hydroxyethylpiperazine-N’-2’-ethanesulfonic Acid buffer and 100 μg/mL gentamycin. Carnosic acid was purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Benzyloxycarbonylvalyl-alanyl-aspartyl fluoromethyl ketone was obtained from Merck millipore (Bedford, MA, USA). N-acetyl-L-cysteine (NAC) was obtained from Calbiochem (San Diego, CA, USA). Anti-CHOP, anti-ATF4 and anti-poly (ADP-ribose) polymerase (PARP) antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Anti-actin antibody and glutathione ethyl ester (GEE) were obtained from Sigma (St. Louis, MO, USA).
2. Flow cytometry analysis
For flow cytometry, the cells were resuspended in 100 μl of phosphate-buffered saline (PBS), and 200 μl of 95% ethanol was added while the cells were being vortexed. Then, the cells were incubated at 4°C for 1 hour, washed with PBS, resuspended in 250 μl of 1.12% sodium citrate buffer (pH 8.4) with 12.5 μg of ribonucleases and incubated for an additional 30 minutes at 37°C. The cellular DNA was then stained by adding 250 μl of a propidium iodide solution (50 μg/ml) to the cells for 30 minutes at room temperature. The stained cells were analyzed by fluorescent-activated cell sorting on a FACScan flow cytometer to determine the relative DNA content, which was based on the red fluorescence intensity.
3. Western blot analysis
For the Western blotting experiments, the cells were washed with cold PBS and lysed on ice in modified radioimmuno-precipitation assay buffer (50 mM Tris-HCl pH 7.4, 1% NP-40, 0.25% Na-deoxycholate, 150 mM NaCl, 1 mM Na3VO4, and 1 mM NaF) containing protease inhibitors (100 μM phenylmethyl-sulfonyl fluoride, 10 μg/ml leupeptin, 10 μg/ml pepstatin, and 2 mM EDTA). The lysates were centrifuged at 10,000 × g for 10 minutes at 4°C, and the supernatant fractions were collected. The proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to Immobilon-P membranes. The specific proteins were detected using an enhanced chemiluminescence Western blotting kit according to the manufacturer’s instructions.
4. The DNA fragmentation assay
The cell death detection ELISA plus kit (Boerhringer Mannheim, Indianapolis, IN, USA) was used to determine the level of apoptosis by detecting fragmented DNA within the nuclei of carnosic acid-treated cells. Briefly, each culture plate was centrifuged for 10 minutes at 200 × g, the supernatant was removed, and the cell pellet was lysed for 30 minutes. Then, the plate was centrifuged again at 200 × g for 10 minutes, and the supernatant that contained the cytoplasmic histone-associated DNA fragments was collected and incubated with an immobilized anti-histone antibody. The reaction products were incubated with a peroxidase substrate for 5 minutes and measured by spectrophotometry at 405 and 490 nm (reference wavelength) with a microplate reader. The signals in the wells containing the substrate alone were subtracted as the background.
5. Asp-Glu-Val-Asp-ase activity assay
To evaluate Asp-Glu-Val-Asp-ase activity, cell lysates were prepared after treatment with carnosic acid. Assays were performed in 96-well microtiter plates by incubating 20 μg of cell lysates in 100 μl of reaction buffer (1% NP-40, 20 mM Tris-HCl, pH 7.5, 137 mM NaCl, 10% glycerol) containing a caspase substrate (Asp-Glu-Val-Asp-chromophore-p-nitroanilide) at 5 μM. Lysates were incubated at 37°C for 2 hours. Thereafter, the absorbance at 405 nm was measured with a spectrophotometer.
6. Measurement of ROS
Intracellular accumulation of ROS was determined using the fluorescent probes 2’, 7’-dichlorodihydrofluorescein diacetate (H2DCFDA). H2DCFDA is commonly used to measure ROS generation.21 Caki cells were pretreated with 5 mM NAC and 2 mM GEE for 30 minutes, and then the cells were incubated with 40 μM carnosic acid for 30 minutes. Cells were stained with the fluorescent dye H2DCFDA and 500 ng/ml Hoechest 33342 (Sigma, St. Louis, MO, USA) for an additional 10 minutes. Then, cells were trypsinized and resuspended in PBS, and fluorescence was measured at specific time intervals with a flow cytometer (Becton-Dickinson, Franklin Lakes, NJ, USA) or was detected by fluorescence microscope (Zeiss, Goettingen, Germany).
7. Small interfering RNA
The ATF4 small interfering RNA (siRNA) duplexes were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). The CHOP and green fluorescent protein (GFP [control]) siRNA duplexes were purchased from Invitrogen (Carlsbad, CA, USA) and had the following sequences: CHOP, GAG CUC UGA UUG ACC GAA UGG UGA A; and GFP, AAG ACC CGC GCC GAG GUG AAG. Cells were transfected with siRNA oligonucleotides using Oligo-fectamine reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s recommendations.
8. Statistical analysis
The data were analyzed using an one-way analysis of variance and post-hoc comparisons (Student-Newman-Keuls) using the Statistical Package for Social Sciences 8.0 software (SPSS Inc., Chicago, IL, USA).
RESULTS
1. Carnosic acid induced apoptosis in human renal carcinoma Caki cells
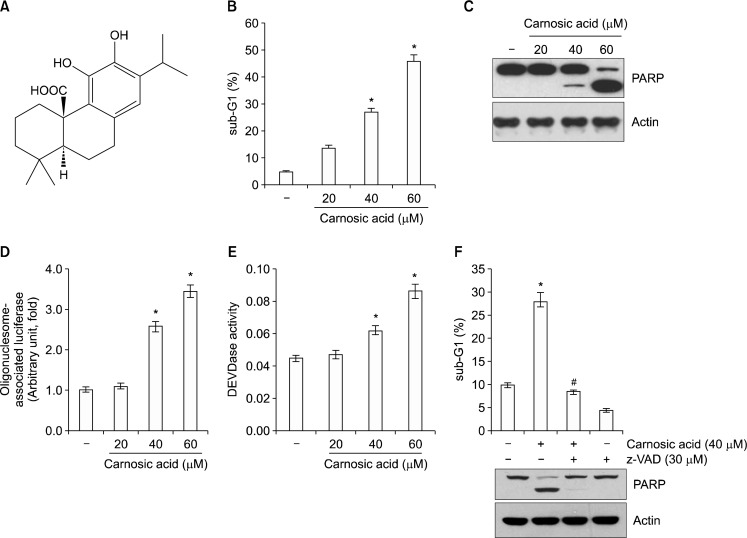
Previous studies reported that carnosic acid has anti-tumor effects and induces apoptosis.1,5,7 Therefore, we investigated whether carnosic acid induces apoptosis in human renal carcinoma Caki cells. To examine this concept, Caki cells were treated with carnosic acid (Fig. 1A). As shown in Figure 1B and C, carnosic acid markedly increased sub-diploid DNA content, sub-G1, population and PARP cleavage, which are apoptosis markers, in a dose-dependent manner. Furthermore, carnosic acid induced cytoplasmic histone-associated DNA fragments (Fig. 1D). Next, we wondered whether caspase activation was involved in carnosic acid-induced apoptosis. Caspase activation was detected in carnosic acid-treated cells (Fig. 1E), and a pan-caspase inhibitor benzyloxycarbonylvalyl-alanyl-aspartyl fluoromethyl ketone, completely blocked apoptosis induced by carnosic acid (Fig. 1F). Therefore, these results suggest that carnosic acid induces apoptosis via caspase activation in human renal carcinoma Caki cells.
Figure 1.
Carnosic acid induces apoptosis in human renal carcinoma Caki cells. (A) The structure of carnosic acid. (B–E) Caki cells were treated with the indicated concentrations of carnosic acid for 24 hours. The sub-diploid DNA content (sub-G1 fraction) was measured by flow cytometry as an indicator of the level of apoptosis (B). The protein expression levels of poly (ADP-ribose) polymerase (PARP) and actin were determined by Western blotting. The level of actin was used as a loading control (C). The cytoplasmic histone-associated DNA fragments were determined by a DNA fragmentation detection kit (D). Caspase activities were determined with colorimetric assays using caspase-3 Asp-Glu-Val-Asp-ase (DEVDase) assay kits (E). (F) Caki cells were treated with 40 μM carnosic acid for 24 hours in the presence or absence of 30 μM benzyloxycarbonylvalyl-alanyl-aspartyl fluoromethyl ketone (z-VAD). The sub-G1 fraction was measured by flow cytometry (E). The protein expression levels of PARP and actin were determined by Western blotting. The level of actin was used as a loading control. The values in (B, D, E, and F) represent the mean ± SD from three independent samples. *P < 0.01 compared to the control. #P < 0.01 compared to the carnosic acid.
2. Reactive oxygen species (ROS) production was associated with carnosic acid-induced apoptosis
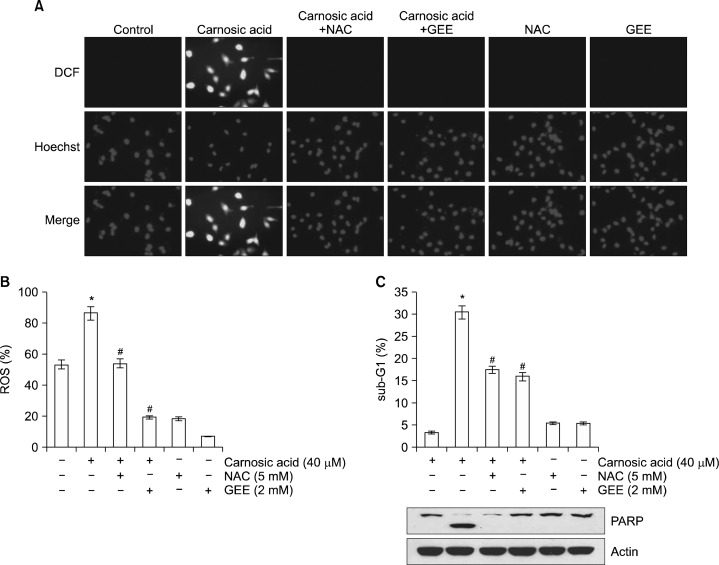
Carnosic acid has been well-known as an anti-oxidant agent, but carnosic acid also increased ROS production.8,22,23 Furthermore, ROS are key signaling molecules to modulate cell death.24,25 To identify relationship between ROS production and apoptosis in carnosic acid-treated cells, we determined the effects of carnosic acid on ROS production. Carnosic acid markedly increased intracellular ROS levels, and ROS scavengers (NAC and GEE) completely blocked ROS production (Fig. 2A and B). Next, we investigated whether ROS mediate carnosic acid-induced apoptosis. Both ROS scavengers decreased carnosic acid-induced apoptosis and PARP cleavage (Fig. 2C). These data suggest that carnosic acid-induced apoptosis is associated with intracellular ROS production.
Figure 2.
Production of ROS is associated with carnosic acid-induced apoptosis. (A and B) Caki cells were pretreated with 5 mM NAC and 2 mM GEE for 30 minutes, and then added with 40 μM carnosic acid for 30 minutes. Cells were stained with the fluorescent dye 2’, 7’-dichlorodihydrofluorescein diacetate (H2DCFDA) and Hoechast 33342 for an additional 10 minutes. Fluorescence was immediately assayed by fluorescence microscope (A) or by flow cytometry (B). (C) Caki cells were pretreated with 5 mM and 2 mM GEE for 30 minutes, and then treated with 40 μM carnosic acid for 24 hours. The level of apoptosis was measured by the sub-diploid DNA content, sub-G1 fraction using flow cytometry. The protein expression levels of poly (ADP-ribose) polymerase (PARP) and actin were determined by Western blotting. The level of actin was used as a loading control. The values in (B and C) represent the mean ± SD from three independent samples. *P < 0.01 compared to the control. #P < 0.01 compared to the carnosic acid. DCF, H2DCFDA.
3. Carnosic acid induced ER
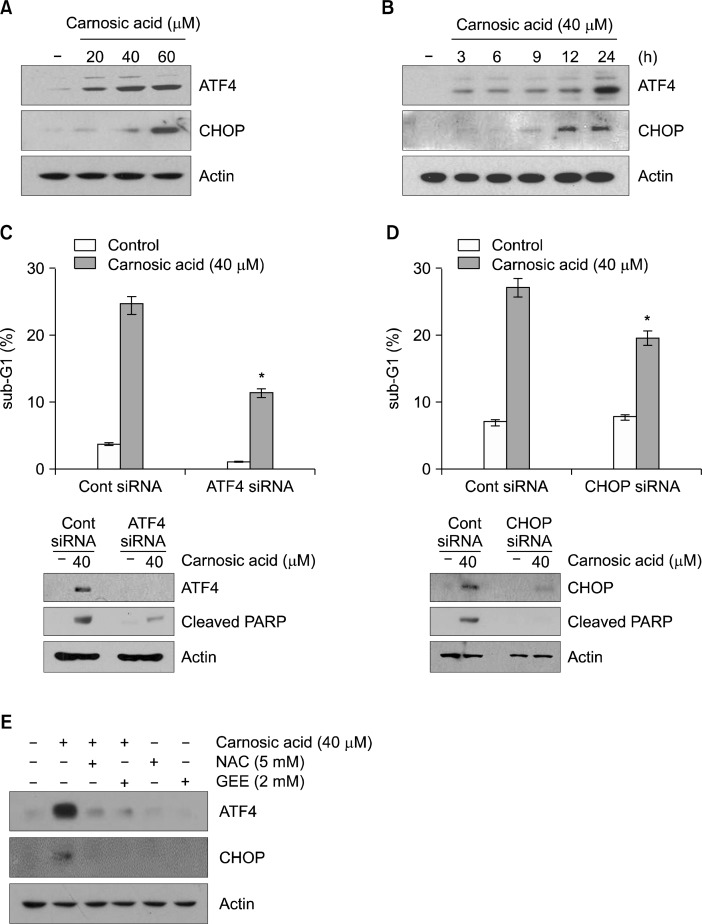
Recent studies have shown that rosemary extracts containing carnosic acid induce ER stress, and excess ER stress could induce apoptosis in cancer cells.26–28 Therefore, we examined whether carnosic acid increases ER stress in human renal carcinoma Caki cells. As shown in Figure 3A, carnosic acid induced ATF4 and CHOP expression in a dose-dependent manner. Up-regulation of ATF4 and CHOP expression was detected within 3 hours and 9 hours, respectively (Fig. 3B). To determine whether up-regulation of ATF4 and CHOP is associated with carnosic acid-induced apoptosis, Caki cells were transfected with ATF4 and CHOP siRNA. Knockdown of ATF4 and CHOP suppressed apoptosis (Fig. 3C and D). Next, we investigated whether ROS is involved in carnosic acid-induced ER stress. ROS scavengers (NAC and GEE) markedly inhibited carnosic acid-induced ATF4 and CHOP expression (Fig. 3E). Therefore, these data suggest that ER stress-mediated ATF4 and CHOP expression play important roles in carnosic acid-induced apoptosis in Caki cells.
Figure 3.
Carnosic acid-induced endoplasmic reticulum stress is involved in apoptosis. (A) Caki cells were treated with the indicated concentrations of carnosic acid for 12 hours. The protein expression levels of activating transcription factor 4 (ATF4), CCAAT/enhancer-binding protein-homologous protein (CHOP), and actin were determined by Western blotting. The level of actin was used as a loading control. (B) Caki cells were treated with 40 μM carnosic acid for the indicated time periods. The protein expression levels of ATF4, CHOP, and actin were determined by Western blotting. The level of actin was used as a loading control. (C and D) Caki cells were transiently transfected with the ATF4 (C) or CHOP (D) small interfering RNA (siRNA), and then treated with 40 μM carnosic acid for 24 hours. The level of apoptosis was measured by the sub-diploid DNA content, sub-G1 fraction using flow cytometry (upper panel). The protein expression levels of ATF4, CHOP, cleaved poly (ADP-ribose) polymerase (PARP) and actin were determined by Western blotting. The level of actin was used as a loading control. (E) Caki cells were pretreated with 5 mM N-acetyl-L-cysteine (NAC) and 2 mM glutathione ethyl ester (GEE) for 30 minutes, and then treated with 40 μM carnosic acid for 24 hours. The protein expression levels of ATF4, CHOP and actin were determined by Western blotting. The actin was used as a loading control. The values in (C and D) represent the mean ± SD from three independent samples. *P < 0.01 compared to the carnosic acid-treated control siRNA (Cont siRNA).
4. Carnosic acid induced apoptosis in other cancer cells, but not normal cells
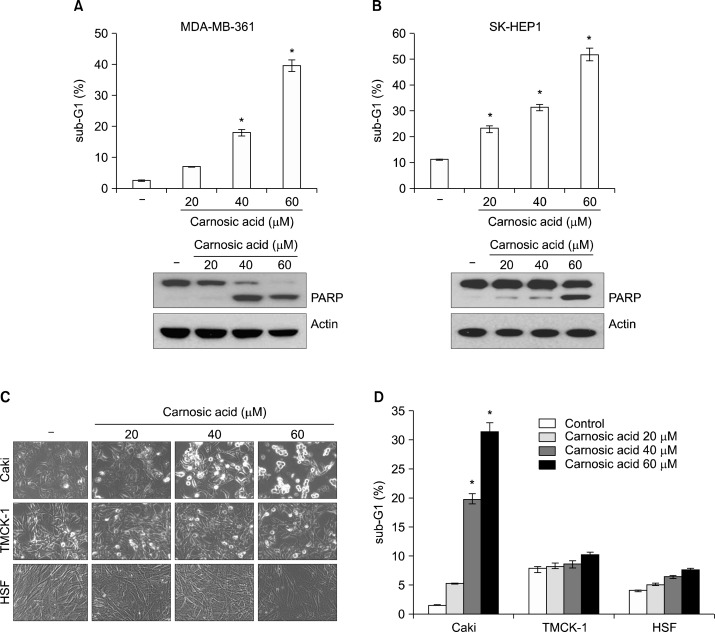
To further confirm an anti-cancer effect of carnosic acid, we investigated whether carnosic acid induces apoptosis in other cancer cells. Carnosic acid induced apoptosis in human breast carcinoma MDA-MB-361 cells and human hepatocellular carcinoma SK-HEP1 cells in a dose dependent manner (Fig. 4A and B). In contrast, carnosic acid induced a cellular morphological change a little bit, but apoptosis was not induced in TMCK-1 and human skin fibroblast non cancer cells (Fig. 4C and D). Taken together, these results indicate that carnosic acid promotes apoptosis in cancer cells, but not normal cells.
Figure 4.
Effect of carnosic acid on apoptosis in other cancer cell lines and normal cells. (A and B) MDA-MB-361 and SK-HEP1 cells were treated with the indicated concentrations of carnosic acid for 24 hours. The level of apoptosis was measured by the sub-diploid DNA content, sub-G1 fraction using flow cytometry (upper panel). The protein expression levels of poly (ADP-ribose) polymerase (PARP) and actin were determined by Western blotting. The level of actin was used as a loading control. (C and D) Caki, TMCK-1, and human skin fibroblast (HSF) cells were treated with the indicated concentrations of carnosic acid for 24 hours. Changes in cellular morphology were visualized by light microscopy (C), and the level of apoptosis was measured by the sub-G1 fraction using flow cytometry (D). The values in (A, B and D) represent the mean ± SD from three independent samples. *P < 0.01 compared to the control.
DISCUSSION
Carnosic acid has been known as an anti-cancer drug, but the underlying mechanism is not well defined. In our study, we identified the mechanism of carnosic acid-induced apoptosis in human renal carcinoma Caki cells. The most important observations made herein are as follows: (i) Carnosic acid induced ROS production. (ii) ROS production is associated with apoptosis. (iii) Carnosic acid induced ER stress. (iv) ER stress-mediated ATF4 and CHOP expression is partially involved in carnosic acid-induced apoptosis.
In previous studies, it is reported that carnosic acid has strong anti-oxidant effect. Carnosic acid effectively scavenges superoxide anion via xanthine/xanthine oxidase and inhibited mitochondrial and microsomal lipid peroxidation.29 These anti-oxidant effects are closely associated with functions of carnosic acid. For examples, carnosic acid (10–20 μM) inhibited migration via suppression of ROS-mediated matrix metalloprotease-9 activation in tumor necrosis factor-α-treated human aortic smooth muscle cells, and carnosic acid (10 μM) inhibited IL-1β-induced cell adhesion molecule expression via suppression of ROS production.30,31 Carnosic acid has been known as a radical scavenger, because carnosic acid transfers hydrogen to radicals. Furthermore, carnosic acid (10 μM) also increased the phase II enzymes, including heme oxygease-1, nicotinamide adenine dinucleotide phosphate quinone oxidoreductase 1, and γ-gluta-mylcysteine synthetase.23 Carnosic acid induces of S-alkylation of the cystein thiol of the Keap1 proteins via binding to a specific Keap1 cysteine residue, and then the Keap1/Nrf2 pathway is activated.32 Nrf2 released from Keap1 by carnosic acid could bind to the antioxidant response element, which is important in expression of phase II enzymes.23 Furthermore, carnosic acid (<1 μM) induced up-regulation of intracellular glutathione levels.33 Glutathione is an important molecule to modulate cellular ROS levels, and is tightly regulated by γ-glutamylcystein ligase (γ-GCL). The γ-GCL is composed of two subunits, GCL catalytic subunit and GCL modifier subunit, and expression of GCL catalytic subunit and GCL modifier subunit is associated with the Nrf2/antioxidant response element pathway. In contrast, carnosic acid induced ROS production. In human neuroblastoma cells, carnosic acid (>30 μM) markedly induced ROS production.8 In our study, carnosic acid (40 μM) also increased intracellular ROS levels, and ROS is involved in carnosic acid-induced apoptosis. Although the mechanism of ROS production by carnosic acid at the high concentrations (> 30 μM) is not clear, action of carnosic acid on ROS production might be different depending on concentrations, cell types, etc. However, our data suggest that ROS production by carnosic acid (40 μM) is important in carnosic acid-induced apoptosis at least in human renal carcinoma Caki cells.
CHOP is a major ER stress-mediated pro-apoptotic transcription factor. CHOP modulates expression of apoptosis-related proteins, including apoptotic Bcl-2 family proteins, death receptor, ER oxidoreduction 1, and tribbles-related protein 3.34 Petiwala et al. reported that rosemary extract, expression of carnosic acid (43%), induced CHOP expression.26 In our study, carnosic acid markedly induced containing ATF4 as well as CHOP (Fig. 3A and B), and carnosic acid-induced apoptosis is associated with up-regulation of ATF4 and CHOP expression (Fig. 3C and D). The mechanism of induction of ER stress by carnosic acid is not clear. However, we found that carnosic acid increased intracellular ROS levels. Redox imbalance is one of causes to induce ER stress. ROS scavengers (NAC and GEE) markedly inhibited ATF4 and CHOP expression in carnosic acid-treated cells (Fig. 3E). Collectively, our results suggest that carnosic acid induced apoptosis through ROS-mediated ER stress induction in the human renal carcinoma Caki cells.
Footnotes
CONFLICTS OF INTEREST
No potential conflicts of interest were disclosed.
REFERENCES
- 1.Rajasekaran D, Manoharan S, Silvan S, Vasudevan K, Baskaran N, Palanimuthu D. Proapoptotic, anti-cell proliferative, anti-inflammatory and anti-angiogenic potential of carnosic acid during 7,12 dimethylbenz[a]anthracene-induced hamster buccal pouch carcinogenesis. Afr J Tradit Complement Altern Med. 2012;10:102–12. doi: 10.4314/ajtcam.v10i1.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Steiner M, Priel I, Giat J, Levy J, Sharoni Y, Danilenko M. Carnosic acid inhibits proliferation and augments differentiation of human leukemic cells induced by 1,25-dihydroxyvitamin D3 and retinoic acid. Nutr Cancer. 2001;41:135–44. doi: 10.1080/01635581.2001.9680624. [DOI] [PubMed] [Google Scholar]
- 3.Pesakhov S, Khanin M, Studzinski GP, Danilenko M. Distinct combinatorial effects of the plant polyphenols curcumin, carnosic acid, and silibinin on proliferation and apoptosis in acute myeloid leukemia cells. Nutr Cancer. 2010;62:811–24. doi: 10.1080/01635581003693082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Visanji JM, Thompson DG, Padfield PJ. Induction of G2/M phase cell cycle arrest by carnosol and carnosic acid is associated with alteration of cyclin A and cyclin B1 levels. Cancer Lett. 2006;237:130–6. doi: 10.1016/j.canlet.2005.05.045. [DOI] [PubMed] [Google Scholar]
- 5.Barni MV, Carlini MJ, Cafferata EG, Puricelli L, Moreno S. Carnosic acid inhibits the proliferation and migration capacity of human colorectal cancer cells. Oncol Rep. 2012;27:1041–8. doi: 10.3892/or.2012.1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Park SY, Song H, Sung MK, Kang YH, Lee KW, Park JH. Carnosic Acid Inhibits the Epithelial-Mesenchymal Transition in B16F10 Melanoma Cells: A Possible Mechanism for the Inhibition of Cell Migration. Int J Mol Sci. 2014;15:12698–713. doi: 10.3390/ijms150712698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kar S, Palit S, Ball WB, Das PK. Carnosic acid modulates Akt/IKK/NF-kappaB signaling by PP2A and induces intrinsic and extrinsic pathway mediated apoptosis in human prostate carcinoma PC-3 cells. Apoptosis. 2012;17:735–47. doi: 10.1007/s10495-012-0715-4. [DOI] [PubMed] [Google Scholar]
- 8.Tsai CW, Lin CY, Lin HH, Chen JH. Carnosic acid, a rosemary phenolic compound, induces apoptosis through reactive oxygen species-mediated p38 activation in human neuroblastoma IMR-32 cells. Neurochem Res. 2011;36:2442–51. doi: 10.1007/s11064-011-0573-4. [DOI] [PubMed] [Google Scholar]
- 9.Gething MJ, Sambrook J. Protein folding in the cell. Nature. 1992;355:33–45. doi: 10.1038/355033a0. [DOI] [PubMed] [Google Scholar]
- 10.Ellgaard L, Molinari M, Helenius A. Setting the standards: quality control in the secretory pathway. Science. 1999;286:1882–8. doi: 10.1126/science.286.5446.1882. [DOI] [PubMed] [Google Scholar]
- 11.Szegezdi E, Logue SE, Gorman AM, Samali A. Mediators of endoplasmic reticulum stress-induced apoptosis. EMBO Rep. 2006;7:880–5. doi: 10.1038/sj.embor.7400779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rasheva VI, Domingos PM. Cellular responses to endoplasmic reticulum stress and apoptosis. Apoptosis. 2009;14:996–1007. doi: 10.1007/s10495-009-0341-y. [DOI] [PubMed] [Google Scholar]
- 13.Malhotra JD, Kaufman RJ. The endoplasmic reticulum and the unfolded protein response. Semin Cell Dev Biol. 2007;18:716–31. doi: 10.1016/j.semcdb.2007.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Walter P, Ron D. The unfolded protein response: from stress pathway to homeostatic regulation. Science. 2011;334:1081–6. doi: 10.1126/science.1209038. [DOI] [PubMed] [Google Scholar]
- 15.Urano F, Wang X, Bertolotti A, Zhang Y, Chung P, Harding HP, et al. Coupling of stress in the ER to activation of JNK protein kinases by transmembrane protein kinase IRE1. Science. 2000;287:664–6. doi: 10.1126/science.287.5453.664. [DOI] [PubMed] [Google Scholar]
- 16.Woehlbier U, Hetz C. Modulating stress responses by the UPRosome: a matter of life and death. Trends Biochem Sci. 2011;36:329–37. doi: 10.1016/j.tibs.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 17.Huang H, Hua X, Liu N, Li X, Liu S, Chen X, et al. Anacardic acid induces cell apoptosis associated with induction of ATF4-dependent endoplasmic reticulum stress. Toxicol Lett. 2014;228:170–8. doi: 10.1016/j.toxlet.2014.05.012. [DOI] [PubMed] [Google Scholar]
- 18.Armstrong JL, Flockhart R, Veal GJ, Lovat PE, Redfern CP. Regulation of endoplasmic reticulum stress-induced cell death by ATF4 in neuroectodermal tumor cells. J Biol Chem. 2010;285:6091–100. doi: 10.1074/jbc.M109.014092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saito S, Takahashi S, Takagaki N, Hirose T, Sakai T. 15-Deoxy-Delta(12,14)-prostaglandin J2 induces apoptosis through activation of the CHOP gene in HeLa cells. Biochem Biophys Res Commun. 2003;311:17–23. doi: 10.1016/j.bbrc.2003.09.161. [DOI] [PubMed] [Google Scholar]
- 20.Lim JH, Park JW, Choi KS, Park YB, Kwon TK. Rottlerin induces apoptosis via death receptor 5 (DR5) upregulation through CHOP-dependent and PKC delta-independent mechanism in human malignant tumor cells. Carcinogenesis. 2009;30:729–36. doi: 10.1093/carcin/bgn265. [DOI] [PubMed] [Google Scholar]
- 21.Lebel CP, Ischiropoulos H, Bondy SC. Evaluation of the probe 2’,7’-dichlorofluorescin as an indicator of reactive oxygen species formation and oxidative stress. Chem Res Toxicol. 1992;5:227–31. doi: 10.1021/tx00026a012. [DOI] [PubMed] [Google Scholar]
- 22.Danilenko M, Wang Q, Wang X, Levy J, Sharoni Y, Studzinski GP. Carnosic acid potentiates the antioxidant and prodifferentiation effects of 1alpha,25-dihydroxyvitamin D3 in leukemia cells but does not promote elevation of basal levels of intracellular calcium. Cancer Res. 2003;63:1325–32. [PubMed] [Google Scholar]
- 23.Satoh T, Izumi M, Inukai Y, Tsutsumi Y, Nakayama N, Kosaka K, et al. Carnosic acid protects neuronal HT22 Cells through activation of the antioxidant-responsive element in free carboxylic acid- and catechol hydroxyl moieties-dependent manners. Neurosci Lett. 2008;434:260–5. doi: 10.1016/j.neulet.2008.01.079. [DOI] [PubMed] [Google Scholar]
- 24.Wu CC, Bratton SB. Regulation of the intrinsic apoptosis pathway by reactive oxygen species. Antioxid Redox Signal. 2013;19:546–58. doi: 10.1089/ars.2012.4905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Matés JM, Segura JA, Alonso FJ, Márquez J. Oxidative stress in apoptosis and cancer: an update. Arch Toxicol. 2012;86:1649–65. doi: 10.1007/s00204-012-0906-3. [DOI] [PubMed] [Google Scholar]
- 26.Petiwala SM, Berhe S, Li G, Puthenveetil AG, Rahman O, Nonn L, et al. Rosemary (Rosmarinus officinalis) extract modulates CHOP/GADD153 to promote androgen receptor degradation and decreases xenograft tumor growth. PLoS One. 2014;9:e89772. doi: 10.1371/journal.pone.0089772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Choi MJ, Park EJ, Min KJ, Park JW, Kwon TK. Endoplasmic reticulum stress mediates withaferin A-induced apoptosis in human renal carcinoma cells. Toxicol In Vitro. 2011;25:692–8. doi: 10.1016/j.tiv.2011.01.010. [DOI] [PubMed] [Google Scholar]
- 28.Park JW, Woo KJ, Lee JT, Lim JH, Lee TJ, Kim SH, et al. Resveratrol induces pro-apoptotic endoplasmic reticulum stress in human colon cancer cells. Oncol Rep. 2007;18:1269–73. [PubMed] [Google Scholar]
- 29.Haraguchi H, Saito T, Okamura N, Yagi A. Inhibition of lipid peroxidation and superoxide generation by diterpenoids from Rosmarinus officinalis. Planta Med. 1995;61:333–6. doi: 10.1055/s-2006-958094. [DOI] [PubMed] [Google Scholar]
- 30.Yu YM, Lin HC, Chang WC. Carnosic acid prevents the migration of human aortic smooth muscle cells by inhibiting the activation and expression of matrix metalloproteinase-9. Br J Nutr. 2008;100:731–8. doi: 10.1017/S0007114508923710. [DOI] [PubMed] [Google Scholar]
- 31.Yu YM, Lin CH, Chan HC, Tsai HD. Carnosic acid reduces cytokine-induced adhesion molecules expression and monocyte adhesion to endothelial cells. Eur J Nutr. 2009;48:101–6. doi: 10.1007/s00394-008-0768-x. [DOI] [PubMed] [Google Scholar]
- 32.Satoh T, Kosaka K, Itoh K, Kobayashi A, Yamamoto M, Shimojo Y, et al. Carnosic acid, a catechol-type electrophilic compound, protects neurons both in vitro and in vivo through activation of the Keap1/Nrf2 pathway via S-alkylation of targeted cysteines on Keap1. J Neurochem. 2008;104:1116–31. doi: 10.1111/j.1471-4159.2007.05039.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen JH, Ou HP, Lin CY, Lin FJ, Wu CR, Chang SW, et al. Carnosic acid prevents 6-hydroxydopamine-induced cell death in SH-SY5Y cells via mediation of glutathione synthesis. Chem Res Toxicol. 2012;25:1893–901. doi: 10.1021/tx300171u. [DOI] [PubMed] [Google Scholar]
- 34.Gorman AM, Healy SJ, Jäger R, Samali A. Stress management at the ER: regulators of ER stress-induced apoptosis. Pharmacol Ther. 2012;134:306–16. doi: 10.1016/j.pharmthera.2012.02.003. [DOI] [PubMed] [Google Scholar]