Figure 1.
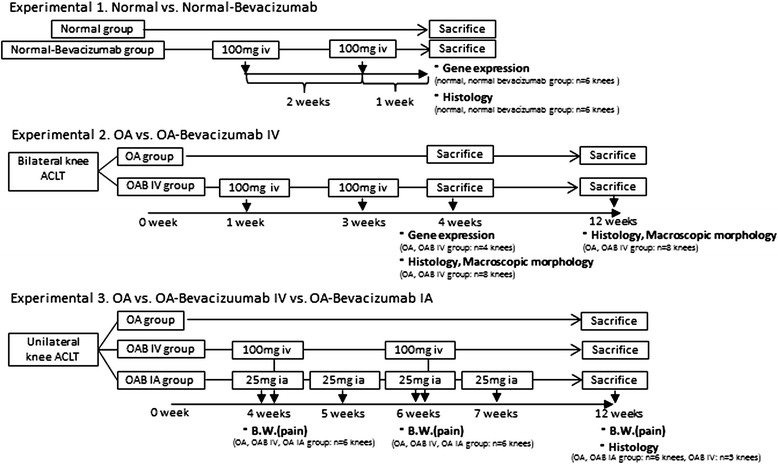
Study outline. This study comprised three experiments to assess the effects of bevacizumab in normal and rabbits with osteoarthritis (OA). Experiment 1: effects of bevacizumab treatment on normal articular cartilage, synovium, and subchondral bone were examined in 12 rabbits. Six normal (untreated) rabbits were given bevacizumab intravenously (iv) on day 1 and at 2 weeks (normal bevacizumab group, n = 12 knees), and the other six normal (untreated) rabbits were used as normal controls (normal group, n = 12 knees). All 12 rabbits were evaluated after 3 weeks to examine gene expression (normal bevacizumab group, n = 6 knees; normal group, n = 6 knees) and histology (normal bevacizumab group, n = 6 knees; normal group, n = 6 knees). Experiment 2: 20 rabbits were given bilateral anterior cruciate ligament transection (ACLT): 10 rabbits were given bevacizumab intravenously 1 week and 3 weeks after ACLT (OAB IV group), and 10 rabbits served as untreated OA controls (OA group). Rabbits were evaluated 4 weeks (n = 6 rabbits/group) and 12 weeks (n = 4 rabbits/group) after ACLT. Experiment 3: all rabbits were given unilateral knee ACLT. Intra-articular bevacizumab (25 mg) was injected 4 weeks after ACLT, then weekly until 7 weeks (OAB IA group). Intravenous bevacizumab (100 mg) was injected 4 and 6 weeks after ACLT (OAB IV group). Rabbits were evaluated 4, 6, and 12 weeks after ACLT. As the comparison, untreated unilateral ACLT rabbits were examined at the same times (OA group).
