Figure 2.
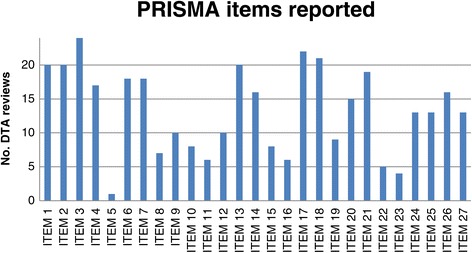
PRISMA items reported by DTA reviews about dementia. Notes: Item 1 = Identify the report as a systematic review; Item 2 = Provide a structured summary; Item 3 = Describe the rationale of the review; Item 4 = Provide an explicit statement of questions being addressed; Item 5 = Indicate if a review protocol exists; Item 6 = Specify study characteristics; Item 7 = Describe all information sources in the search and date last searched; Item 8 = Present full electronic search strategy; Item 9 = State the process for selecting studies; Item 10 = Describe method of data extraction; Item 11 = List and define all variables for which data were sought; Item 12 = Describe methods used for assessing risk of bias of individual studies; Item 13 = State the principal summary measures; Item 14 = Describe the methods of handling data and combining results; Item 15 = Specify any assessment of risk of bias that may affect the evidence; Item 16 = Describe methods of additional analyses; Item 17 = Give numbers of studies screened, assessed for eligibility, and included in the review; Item 18 = For each study, present characteristics for which data were extracted; Item 19 = Present data on risk of bias of each study; Item 20 = For all outcomes present simple summary data, effect estimates and confidence intervals; Item 21 = Present the main results of the review; Item 22 = Present results of any assessment of risk of bias across studies; Item 23 = Provide results of additional analyses; Item 24 = Summarize the main findings; Item 25 = Discuss limitations at study and outcome level and at review-level; Item 26 = Provide a general interpretation of the results and implications for future research; Item 27 = Describe sources of funding for the systematic review.
