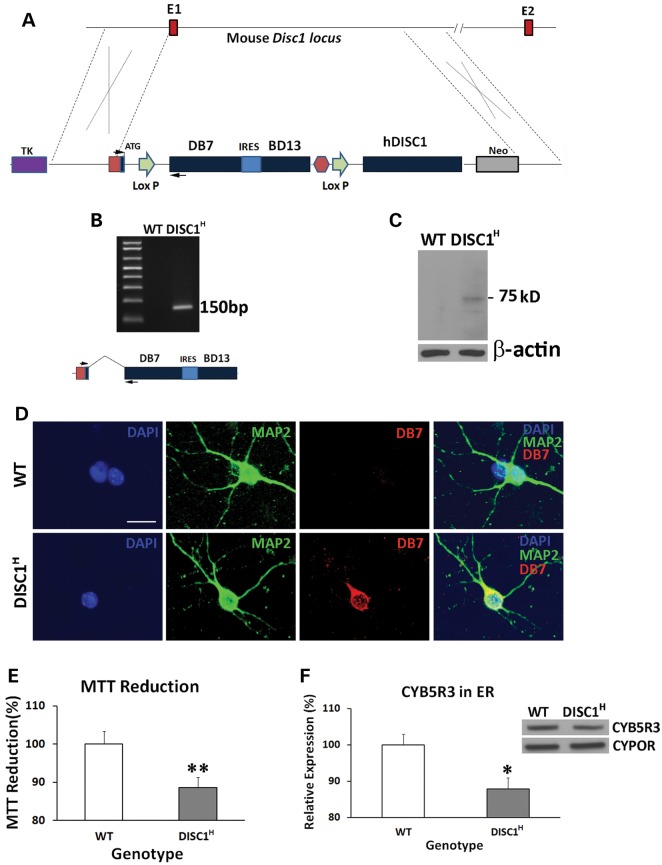
Figure 5.
Generation of humanized DISC1-Boymaw Mice. (A) Both the DISC1-Boymaw and the Boymaw-DISC1 fusion genes were knocked-in to replace mouse endogenous disc1 gene. E1 and E2 are exon 1 and 2 of mouse disc1 gene. (B) RT-PCR analysis of the expression of the DB7 fusion genes in humanized DISC1-Boymaw mice (DISC1H). The two PCR primers were localized in exon 1 and DB7, respectively (arrows). The expression of the bi-cistronic gene generated a 150 bp cDNA fragment after RT-PCR. Correct splicing was confirmed after sequencing. (C) Western blot analysis of the DB7 fusion protein in cultured primary neurons. Expression of the DISC1-Boymaw (DB7) fusion proteins was detected from the primary neurons isolated from the humanized DISC1-Boymaw mice. The 75 kDa protein band is very close to predicted molecular size (∼73 kDa). (D) Immunocytochemical staining of the DB7 fusion proteins in the primary neurons. Primary neurons were isolated from postnatal Day 1 mice, and cultured 4 days in vitro before immunostaining. Co-localization between MAP2 and DB7 proteins was observed. Scale bar: 20 µm. (E) Decreased MTT reduction in brain homogenate of neonatal heterozygous mice (DISC1H) (n = 14) in comparison with wild-type control littermates (n = 12) after normalization with total amount of proteins (t(24) = 2.984, P < 0.01, unpaired, two-tailed student's t-test). (F) Reduced expression of Cyb5r3 in the ER isolated from the brains of neonatal DISC1-Boymaw mice (n = 16) and wild-type littermates (n = 18) after normalization with ER marker Cypor (unpaired, two-tailed student's t-test, t(32) = 2.58, P < 0.05). Error bar: SEM. (*P < 0.05, **P < 0.01).