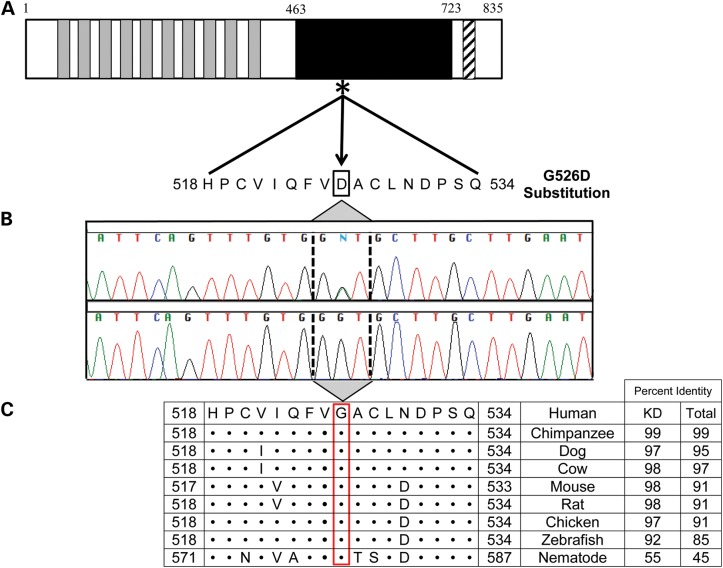
Figure 3.
Location and conservation of TNNI3K-G526D mutation. (A) The protein topology is shown for TNNI3K, including 10 functional ankyrin repeat domains (gray), a serine-rich domain (diagonal hatch lines) and a single functional kinase domain (black) where the heterozygous c.1577 G > A resides (asterisk). (B) Sanger sequencing verified the mutation, which resulted in a G526D substitution. (C) Conservation of this residue is illustrated whereby • indicates identical residues. Overall, the amino acid sequence of TNNI3K is highly conserved with the amino acid identity to the human TNNI3K ortholog indicated for both the kinase domain (KD) and the entire protein.