Figure 3. Fitting of eNOS homology model into the reconstructions human eNOS holoenzyme.
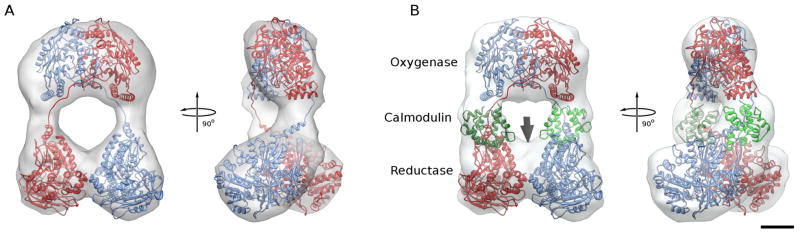
A. Fit of final model of the eNOS homo dimer into the reconstruction of eNOS in the absence of CaM. One monomer is shown in red, the other in blue. Same views as in Figure 2A.
B. Fit of final model of the eNOS homo dimer with bound CaM into the reconstruction of eNOS in the presence of CaM. The two CaMs are shown in different shades of green and occupy the extra density seen in Figure 2B. There is some unoccupied density near the place where the C-termini of the reductase domains interact (arrow), indicating that the C-terminal residues disordered in the crystal structure are more ordered in this conformation and possibly contribute to the stabilization of the reductase domain dimer interface.
