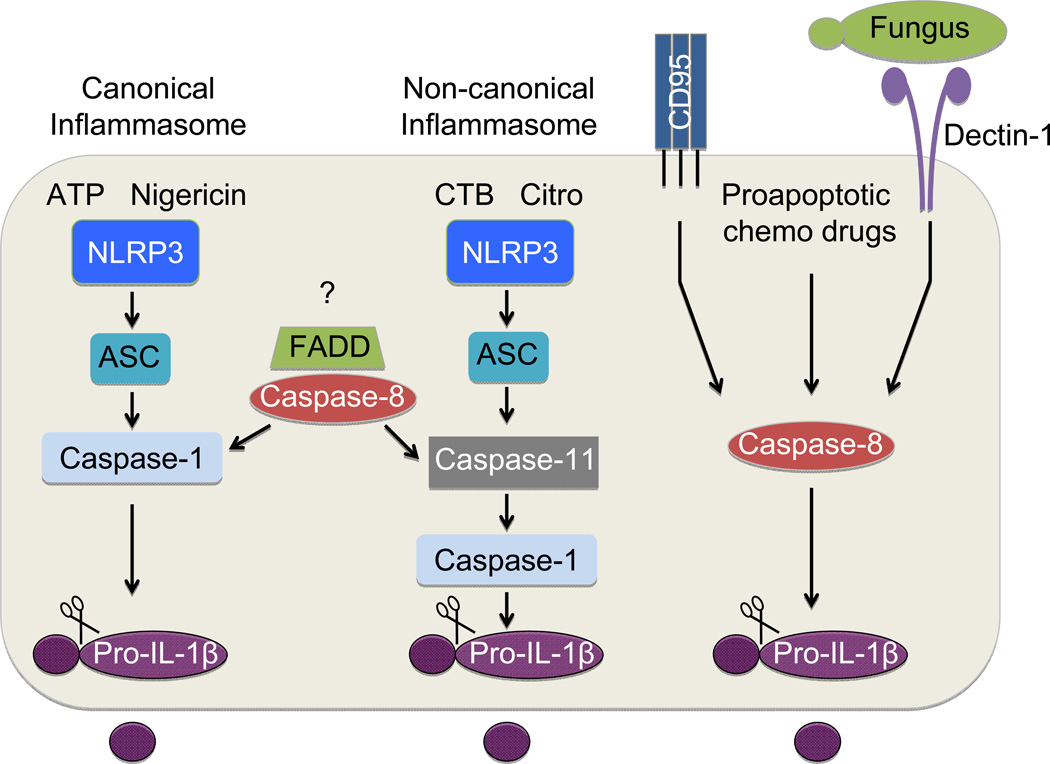
Figure 2. Pathways involved in caspase-mediated IL-1β maturation.
IL-1β requires cleavage to elicit its biological activity and to promote its secretion. Multiple caspase-dependent pathways have been identified to promote IL-1β maturation. The best characterized of these pathways involves caspase-1 activation in inflammasome complexes. Recognition of pathogen- or danger-associated molecular patterns (PAMPs and DAMPs, respectively) by NOD-like receptors (NLRs) or AIM2-like receptors (ALRs) promotes the recruitment of caspase-1 into a multiprotein complex with the help of the adaptor protein ASC. Inflammasome complex formation orchestrates the self-cleavage and activation of caspase-1. Active caspase-1 subsequently cleaves the pro-form of IL-1β. Inflammasomes were originally thought to only require participation from a cytosolic sensor molecule, the adaptor protein ASC and caspase-1. However recent studies suggest that additional caspases and signaling molecules can directly impinge on pathways that coordinate IL-1β maturation. For instance, caspase-11 is required for efficient downstream caspase-1 and IL-1β activation in complexes that are now referred to as non-canonical inflammasomes. Caspase-11 is essential for inflammasome-induced IL-1β production in response to cholera toxin B and citrobacter rodentium. In contrast, other activators of inflammasome-dependent IL-1β maturation such as ATP and nigericin can trigger IL-1β cleavage via canonical inflammasomes in a fashion that is NLR/ASC/caspase-1 dependent and caspase-11 independent. The traditional cell death molecules, caspase-8 and FADD, have also been established to play major regulatory roles in both canonical and non-canonical inflammasome activation. However the upstream regulators of FADD and caspase-8 in these pathways still remain to be identified. Caspase-8 can also cleave IL-1β independently of caspase-1/11, and this has been suggested to contribute to IL-1β production that occurs in response to engagement of the death receptor CD95 (also known as FAS), fungal infection, and following treatment of cells with proapoptotic chemotherapeutic drugs.