Figure 3.
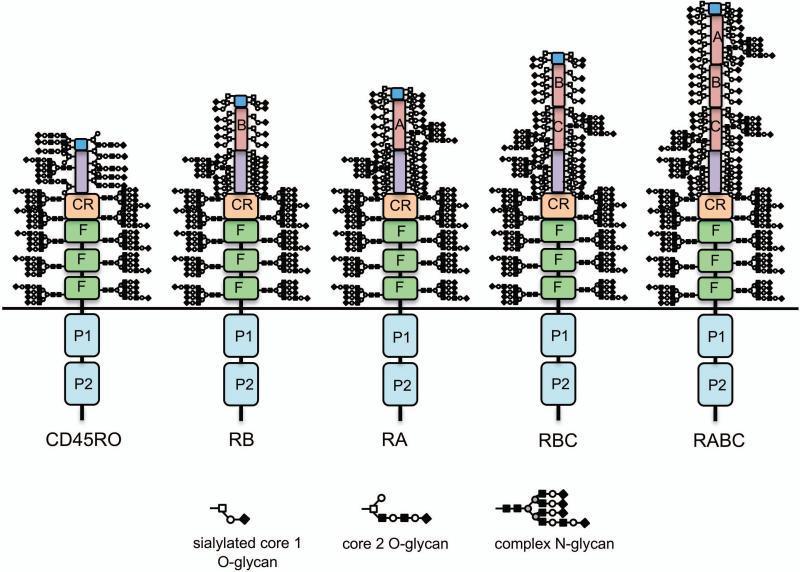
There are five isoforms of CD45 on human lymphocytes due to alternative splicing. Intracellularly, all isoforms of CD45 have tandem phosphatase domains (P1 and P2; only P1 is enzymatically active). Extracellularly, all isoforms have three fibronectin type III repeats (F), a cysteine-rich region (CR), and a terminal region. The F, CR, and terminal regions are the primary sites of N-linked glycosylation. Alternative splicing of exons 4, 5, and 6 generates up to three additional extracellular domains—termed A, B, and C, respectively—and a total of five isoforms of CD45: RO (with no alternatively spliced domains), RB (exon 5), RA (exon 4), RBC (exons 5 and 6), and RABC (exons 4-6). The extended region and alternatively spliced regions are the primary sites of O-linked glycosylation, although RA and RC also have canonical sites of N-linked glycosylation. The schematics of the five isoforms of CD45 depict glycans based on the following assumptions: 1) The molecular weights of CD45RO, RB, RA, RBC, and RABC are 180, 190, 210, and 230, respectively; 2) Each N-glycan is complex and contributes ~4 kDa to the overall molecular weight; RO has core 2 O-glycans, each contributing ~1.5 kDa to the overall molecular weight; RB, RA, and RBC have sialylated core 1 O-glycans, each contributing ~0.7 kDa to the overall molecular weight; 3) All N-glycan sites in the F, CR, and extended regions are occupied in all isoforms; 4) Some O-glycan sites may overlap between isoforms, but the total number of O-glycans should be higher for larger isoforms (i.e., RO should have the least number of O-glycans, while RABC should have the most O-glycans); 5) Domains with more serine and threonine residues should have more potential O-glycosylation sites (i.e., RB has the least and RA has the most); and 6) The alternatively spliced domains A, B, and C should have O-glycan occupancy approximately proportional to the number of serine and threonine residues contained in the polypeptide sequences.