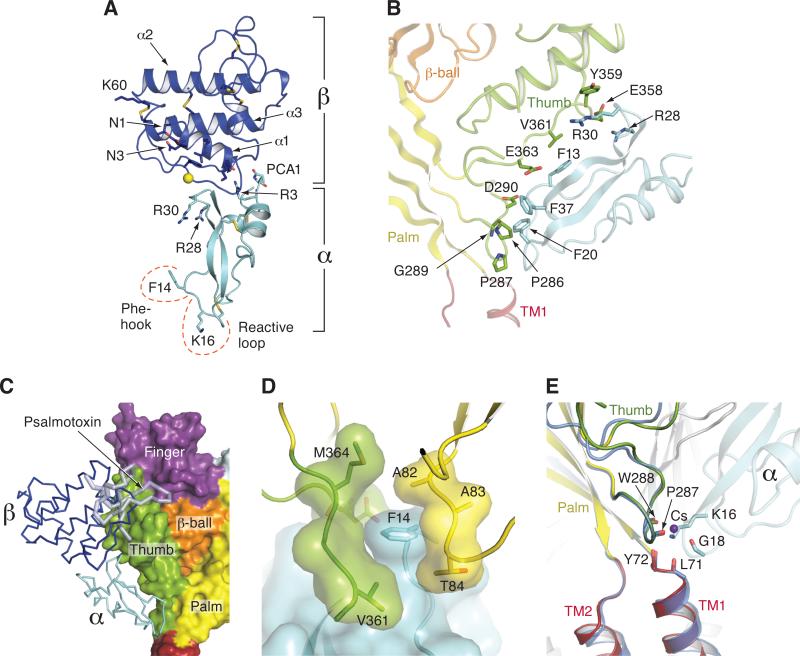
Figure 2.
Structures of MitTx and illustration of key residues and interactions. (A) Structure of MitTx derived from the complex with Δ13. In the heterodimeric α/β toxin complex, each subunit buries ~500 Å of solvent accessible surface area at the subunit interface. Residues near the N and C- termini of the α subunit play particularly important roles in the heterodimeric complex, forming helix-capping contacts to the C-terminus of the α1 helix of the β subunit, as well as mediating interactions with the β-wing domain. The α subunit, depicting the key Phe 14 and Lys 16 residues, along with the 3 disulfide bonds. (B) Illustration of residues that participate in extensive interactions between the α subunit of MitTx (cyan) and residues on the thumb domain (green) with portions of the Δ13 palm domain (light yellow) and β-ball (light orange) also shown. Phe 13, 20 and 37 supplement the interactions between α and the thumb of Δ13, and together with Arg 28 and 30, make interactions with residues on the α4 and α5 helices. (C) The binding site of MitTx (blue and cyan) overlaps with the psalmotoxin (light blue) binding site. Δ13 is shown in surface representation and toxins are in ribbon representation. (D) View of the subunit interface separated by Phe 14 of the α subunit, which serves as a ‘flange’ of the MitTx ‘churchkey.’ (E) View of the ‘wrist’ region following superposition of Cα positions of residues 285-290 and 70-74 of Δ13-MitTx and low pH Δ13-PcTx1 soaked in Cs+. Coordination of the ammonium group of Lys 16 is similar to the carbonyl oxygen coordination with Cs+ in the Δ13-PcTx1 structure. See also Figure S2.