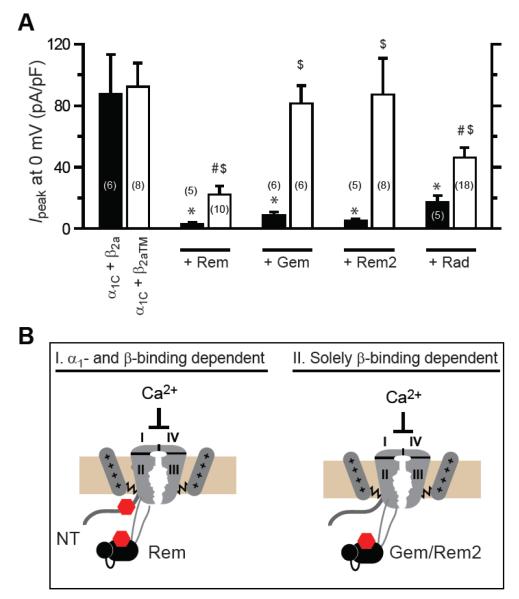
Figure 4. Distinct RGKs differentially use β-binding-dependent and β-binding-independent mechanisms to inhibit CaV1.2 channels.
(A) Impact of distinct RGKs on wild-type (α1C+β2a) and mutant (α1C+β2aTM) CaV1.2 channels. *, #, $ P < 0.05 when compared to α1C+β2a, α1C+ β2aTM, or α1C+β2a+RGK, respectively, using two-tailed unpaired Student’s t test. (B) Cartoon showing dichotomy in determinants used by distinct RGKs to inhibit CaV1.2 channels. Rem can inhibit CaV1.2 by both β-binding-dependent and α1-binding-dependent mechanisms. Gem and Rem2 inhibit CaV1.2 solely using a β-binding-dependent mechanism. The stoichiometry of Rem binding to the CaV1.2 complex has not been investigated. One possibility is that two Rem proteins can simultaneously associate with a single CaV1.2 channel. Alternatively, it is also possible that within a single CaV1.2 channel complex, the interaction of Rem with α1C N-terminus or β subunit is mutually exclusive, thus ensuring a 1:1 binding stoichiometry. Figure reproduced from [17].