Figure 2.
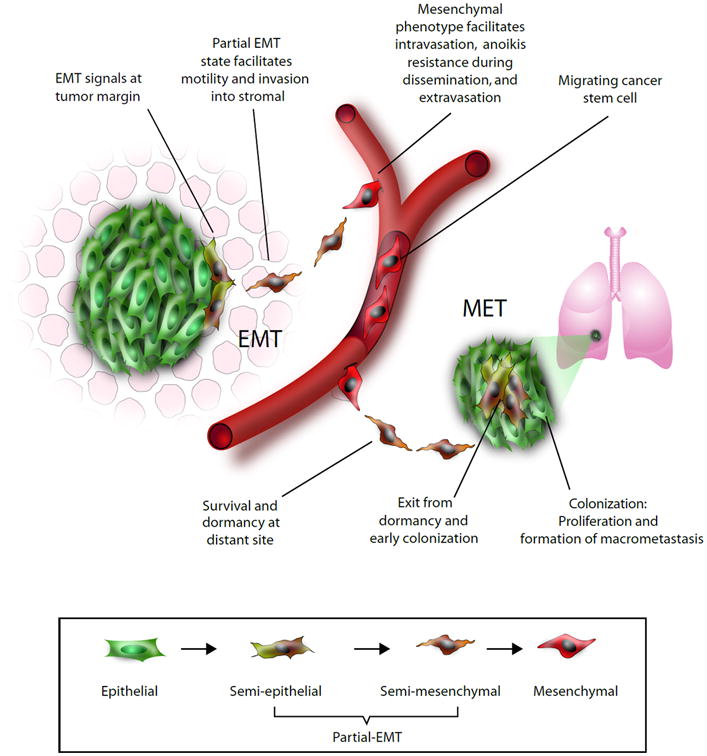
Epithelial-mesenchymal plasticity allows cancer cells to undergo functional adaptations during the invasion-metastasis cascade. In response to EMT-promoting signals, a subpopulation of epithelial cells at the invasive edge of the tumor may lose epithelial traits. As these cells detach further from the bulk of the tumor, they become less exposed to epithelial signals and acquire more mesenchymal properties in the presence of EMT signals supplied by stromal cells121–124. The metastable mesenchymal cells are suited for invasion into surrounding tissues. A fully mesenchymal phenotype facilitates intravasation into blood capillaries or draining lymphatic vessels. In some instances, this process may be aided by macrophages125. The disseminating cancer cell is also more resistant to environmental and genotoxic stresses, a characteristic that is crucial for survival in circulation126. After arrival at a distant organ, the mesenchymal phenotype facilitates extravasation and invasion into the foreign tissue. Here disseminated cells are exposed to signals different from those of the primary tumor, and the mesenchymal state may confer survival advantages to single cancer cells or alternatively may support long-term dormancy127. When the appropriate contextual signals become available, disseminated cells may undergo an MET and gradually reacquire epithelial properties such as rapid proliferative capabilities16,17. Epithelial signals are reinforced through autocrine and paracrine signals, resulting in the stabilization of an epithelial phenotype. This facilitates the outgrowth of macrometastases that are composed predominantly of epithelial cells.
