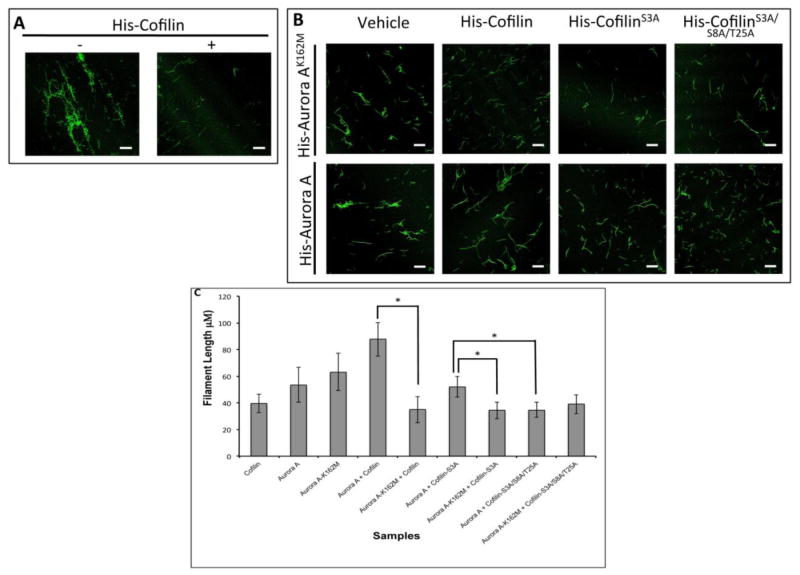
Figure 3. Phosphorylation by Aurora A Reduced Actin Depolymerizing Activity of Cofilin.
A: Images showing the depolymerization of actin by Cofilin. Decreased Phalloidin staining of F-actin could be noted in the presence of His-Cofilin. B: Recombinant His-Cofilin, His-CofilinS3A, or His-CofilinS3A/S8A/T25A mutants were in vitro phosphorylated by His-Aur-AK162M (top panels) or His-Aur-A (bottom panels) and incubated with polymerized actin and stained with Phalloidin. C: Quantification of actin filament length from B. Incubation with phosphorylated His-Cofilin or His-CofilinS3A mutant by inactive Aur-A reduced Phalloidin staining compared to His-cofilin or His-CofilinS3A phosphorylated with active Aur-A. Incubation with phosphorylated His-CofilinS3A/S8A/T25A by active Aur-A partially retained Cofilin activity as noted by shorter fragments of Phalloidin stained F actin compared to His-Cofilin or His-CofilinS3A. Data is representative of ten longest actin filaments each in 15 fields of two independent experiments. Scale bar: 25μm, *p<0.05